Autoimmune thyroid diseases (AITD), including Graves’ disease (GD) (hyperthyroidism) and Hashimoto’s thyroiditis (HT) (hypothyroidism) are the most common form of thyroid dysfunction, with HT being the most frequent. In HT and GD the immunological mechanisms involved are closely related, while the phenotypes probably differ because of the specific type of immunological response that occurs.[1]
Signs and symptoms of Hashimoto's thyroiditis and Graves disease[36-38]Hashimoto's thyroiditis (HT)The most common form of autoimmune thyroiditis. Generally responsible for hypothyroid symptoms, however it is possible for HT symptoms to swing between hypothyroidism and hyperthyroidism. In HT autoantibodies decrease precursors necessary for thyroid hormone production, thereby causing hypothyroidism. Common symptoms: Weight gain, fatigue, dry skin, hair loss, intolerance to cold, and constipation. Thyroid autoantibodies which may be elevated: TPOab, Tgab Graves’ disease (GD)The most common cause of hyperthyroidism. It is triggered by the immune system producing autoantibodies that stimulate TSH receptors (in the thyroid gland), thereby increasing the size of the gland and increasing thyroid hormone production and causing hyperthyroidism. Common symptoms: Sweating, rapid heart rate, anxiety, tremors, fatigue, difficulty sleeping, sudden weight loss, and protruding eyes. Thyroid autoantibodies which may be elevated: TPOab,TRAb, TSHR Ab, TSI |
These conditions are characterised by circulating antibodies and lymphocyte infiltration. Most commonly, thyroid peroxidase antibodies (TPOab) and/or thyroglobulin antibodies (Tgab) are present. There is also generally an inflammatory immune profile present with elevations in Th17 and Th1 cells, together with Treg suppression.
As with other autoimmune conditions, AITD risk has a genetic component, whereby additional environmental and existential factors provide provocation or triggers which ultimately lead to disease development.[2] Environmental risk factors identified to contribute to AITD include age and gender (with a higher incidence among females), iodine consumption, infections, stress, toxicity (e.g. smoking, high alcohol consumption, polychlorinated biphenyls, mercury exposure) and certain nutritional depletions.[2,3]
Ensuring the absence of nutritional deficiencies is recommended as one of the first steps in managing the wellbeing of an AITD patient. Deficiencies of iodine (or iodine excess), iron, selenium and zinc have been found to impair thyroid function, whilst other deficiencies, such as that of vitamins A, C, B6, B5 and B1, phosphorous, magnesium, potassium, sodium and chromium, have all been observed in thyroid patients.[4] Improving diet quality is therefore recommended.
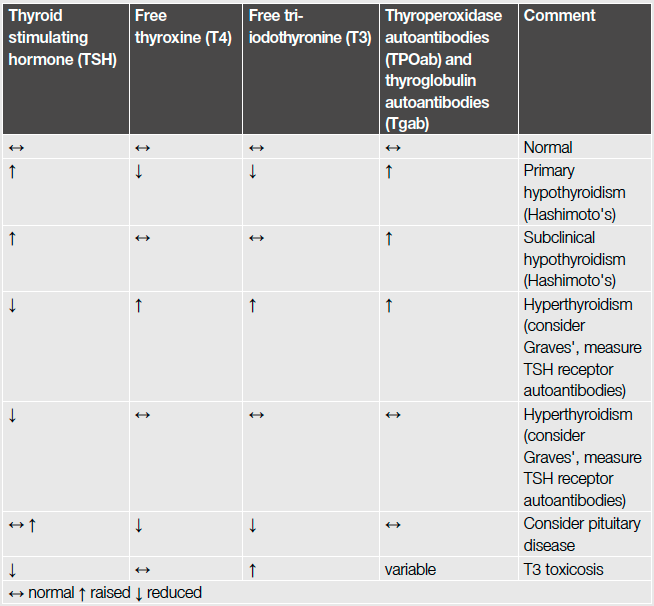
Common results of thyroid function tests[35]
The role of iodine
Iodine is essential for thyroid hormone synthesis, and is well known for its role in healthy thyroid function. Avoidance of deficiency is important in the prevention of thyroid underactivity, however long-term excessive intake is recommended against also. This is the result of findings indicating increased iodine consumption amongst communities resulting in greater prevalence of TPOab and hypothyroidism.[3]
Iodine status and/or investigation of dietary and supplemental consumption of iodine is recommended to ensure the absence of excess or insufficiency.
Antioxidant selenium and alpha lipoic acid
Not only is selenium essential for normal thyroid function via its contribution to the deiodination enzymes involved in converting T4 to the active thyroid hormone T3, low selenium concentrations have been found to cause autoimmune processes in the thyroid gland.[3] As a result research has been conducted on the effectiveness of selenium for the management and progression of AITD (both GD and HT).
In a pilot study on GD patients, selenium therapy used concurrently with routine methimazole treatment (a thyroperoxidase (TPO) inhibitor) was compared to methimazole alone (controls) for 6 months. The addition of selenium was found to assist in achieving better outcomes for patients. Selenium supplementation achieved greater decreases in free T4 and T3, greater increases in TSH and significantly lower anti-TSH-receptor antibodies (TRAb). There was also a higher rate of remission in the selenium group compared to controls, and a higher percentage of patients achieved normal TRAb within the 6 month treatment period.[5]
A common symptom of GD is ophthalmopathy/orbitopathy (protrusion of the eyes). This is suggested to be the result of greater levels of inflammation and oxidative stress which contribute to adipose tissue expansion behind the eyes, linked to the action of inflammatory cytokines which activate orbital fibroblast secretion of glycosaminoglycans, inducing orbital fibrosis and oedema.[6]
Selenium has demonstrated its therapeutic potential as an agent for Graves' orbitopathy.
A dose of selenium (100mcg twice daily) was compared to pentoxifylline (an antiinflammatory agent) and placebo in patients with mild Graves’ orbitopathy. At the 6-month evaluation, treatment with selenium (but not pentoxifylline) was associated with improved quality of life and less eye involvement, and a slowed progression of Graves’ orbitopathy when compared to placebo. Selenium also showed benefit in slowing disease progression, whereas pentoxifylline and placebo did not.[7]
Alpha lipoic acid (ALA) has been shown to be beneficial in orbitopathy also, with suggested mechanisms of action including antioxidant and anti-inflammatory actions which prevent adipose fibroblast expansion behind the eyes.[8]
Furthermore, oxidative stress (resulting from inflammation, toxicity, etc.) is indicated to play a role in the down regulation of T4 to T3 conversion, contributing to hypothyroid symptoms. Via its antioxidant and antiinflammatory actions, ALA has been shown to restore healthy T3 and rT3 levels by downregulating the oxidative stress that interferes with normal deiodination function responsible for healthy T4 to T3 conversion.[9] This suggests the nutrient’s benefits in under activity of thyroid function.
Gluten-free diet
There is a higher prevalence of coeliac disease (CD) in AITD sufferers.[10] A meta-analysis identified that approximately one in 62 patients with AITD have biopsy-verified CD.[11] Given this prevalence it is argued that patients with AITD should be screened for CD and treated accordingly. In the absence of CD, removal of gluten as part of dietary intervention remains a worthy consideration due to research demonstrating the ability of gluten fractions (gliadin) to increase the expression of zonulin. Zonulin regulates tight junction function in the gut. Elevated zonulin (stemming from gluten consumption and other factors in genetically susceptible individuals) contributes to increased intestinal permeability and subsequent lipopolysaccharide (LPS) endotoxin passage into the body.[12,13] LPS trigger the release of Th17 immune cells, which in high numbers facilitate the autoimmune disease process.[14]
In addition to considered gluten removal from the diet, certain probiotic species (e.g. Lactobacillus rhamnosus,[15] L. plantarum,[16,17] L. acidophilus [17] and Bifidobacterium longum)[17] have been demonstrated to reduce zonulin expression and assist in maintenance/restoration of intestinal integrity, while restoration of microbial diversity may also assist in achieving an increase in Treg coupled with the desired decrease in autoimmune and inflammatory Th17 and Th1 (respectively). This alteration to the immune profile can facilitate restoration of immune tolerance, mitigating autoimmune disease activity.
Zinc supplementation also shows promise for attenuation of stress-induced changes in intestinal integrity via specific beneficial effects on tight junction complexes.[18,19]
Vitamin D
Vitamin D is identified to modulate both innate and adaptive immune systems, and low vitamin D levels have been identified as a risk factor for various autoimmune diseases. A recent meta analysis revealed lower blood levels of vitamin D in AITD patients when compared with controls. AITD patients (with GD or HT) were also more likely to have deficiency.[20] Furthermore, a later study supported these findings with vitamin D insufficiency (defined as serum 25(OH)D level <75nmol/L) associated with AITD and HT, especially overt hypothyroidism.[21] Low serum vitamin D levels were also independently associated with high serum TSH levels.
Assessment of vitamin D status is thus recommended, with supplementation prescribed accordingly.
Infections
Evidence is mixed, however it appears that Helicobacter pylori might be a risk factor for AITD and high thyroid volume.[22] Persistent underlying infections such as H. pylori,[23] herpes,[24] Epstein-Barr virus (EBV)[25] and hepatitis C[2] have been hypothesised as potential triggers for autoimmunity and therefore an assessment to rule out their presence may be recommended. Addressing underlying infections may prevent ongoing immune activation, inflammation and oxidative stress that are the hallmarks of autoimmunity.
Sex hormones and thyroid
HT and polycystic ovarian syndrome (PCOS) are closely associated, with a number of studies showing a higher prevalence of HT, elevated TSH, TPOab and Tgab levels in women with PCOS than in controls.[26,27] Furthermore, increased oestrogen and oestrogen/progesterone ratios seem to be directly involved in the observed high TPOab levels in PCOS patients.[27]
Assessing thyroid patients (not only those with concurrent PCOS) for progesterone/ oestrogen imbalance may hence be a worthy consideration in autoimmune patients. In addition, low progesterone and high oestrogen may co-exist with prolactin elevations, another scenario hypothesised as a trigger for autoimmunity.[28,29] It is suggested that excessive prolactin secretion may favour an inflammatory environment, and decrease the suppressor function of Treg cells (which function to maintain immune tolerance), favouring then the expansion of auto-reactive lymphocytes which then favours autoimmune activation.[30]
Vitex agnus castus is suggested to be useful in latent hyperprolactinaemia, with one trial reporting it to be superior to placebo for reducing TRH-stimulated prolactin secretion, normalising a shortened luteal phase, increasing mid-luteal progesterone and 17β-oestradiol levels.[31] Anti-inflammatory/immune-modulating ingredients (e.g. curcumin and vitamin D3) may be indicated to assist in minimising oestrogen elevations, together with zinc, a suggested natural aromatase inhibitor. Detoxification support may also assist in facilitating healthy endogenous- and xenooestrogen metabolism and removal.
It is worth noting that hypothyroidism, via the subsequent rise in thyrotropin releasing hormone (TRH) causes altered follicle stimulating hormone (FSH):luteinising hormone (LH) ratio, and raised dehydroepiandrosterone (DHEA-S) levels. More so, excess thyroid stimulating hormone (TSH) causes stimulation of FSH receptor.[32] Considering this, PCOS sufferers may benefit from screening for hypothyrodisim, and vice versa, to determine if a concurrent dysfunction is exacerbating hormonal imbalances, weight gain, and insulin resistance.
ALA and inositol are emerging as interesting considerations for HT and PCOS (both alone and in combination with pharmaceutical intervention). ALA (suggested dose 400-800mg)33,34 exhibits not only antioxidant and immune-modulating benefits, but also assists mitochondrial function and healthy glycaemic control. Inositol (particularly myo-inositol at 1-2g/day)[33,34] facilitates T3 and insulin function by acting as a ‘second messenger’ within target cells. This nutrient combination may therefore be indicated for patients experiencing insulin resistance, altered metabolism and inflammation associated with HT and/or PCOS.
Key considerations or intervention in AITD:
|
Conclusion
In conclusion, considering each of the factors discussed, together with stress management, may assist patients with AITD. It is worth highlighting that research has demonstrated certain nutritional therapies to enhance the outcomes patients achieve through pharmaceutical drug intervention, however it is recommended to monitor patients closely and consider potential nutrient/drug and nutrient/herb interactions prior to prescription.
References
- Trbojević B, Djurica S. Diagnosis of autoimmune thyroid disease. Srp Arh Celok Lek 2005;133 Suppl 1:25-33. [Abstract]
- Duntas LH. Environmental factors and autoimmune thyroiditis. Nat Clin Pract Endocrinol Metab 2008;4(8):454-460. [Abstract]
- Wiersinga WM. Clinical relevance of environmental factors in the pathogenesis of autoimmune thyroid disease. Endocrinol Metab (Seoul) 2016;31(2):213-222. [Full text]
- Kawicka A, Regulska-Ilow B. Metabolic disorders and nutritional status in autoimmune thyroid diseases. Postepy Hig Med Dosw (Online) 2015;69:80-90. [Full text]
- Wang L, Wang B, Chen SR, et al. Effect of selenium supplementation on recurrent hyperthyroidism caused by Graves' disease: a prospective pilot study. Horm Metab Res 2016;[Epub ahead of print]. [Abstract]
- Dharmasena A. Selenium supplementation in thyroid associated ophthalmopathy: an update. Int J Ophthalmol 2014;7(2):365-375. [Full text]
- Marcocci C, Kahaly GJ, Krassas GE, et al. Selenium and the course of mild Graves' orbitopathy. N Engl J Med 2011;364(20):1920-1931. [Full text]
- Hwang S, Byun JW, Yoon JS, et al. Inhibitory effects of α-lipoic acid on oxidative stress-induced adipogenesis in orbital fibroblasts from patients with Graves ophthalmopathy. Medicine (Baltimore) 2016;95(2):e2497. [Full text]
- Chen K, Yan B, Wang F, et al. Type 1 5'-deiodinase activity is inhibited by oxidative stress and restored by alpha-lipoic acid in HepG2 cells. Biochem Biophys Res Commun 2016;472(3):496-501. [Abstract]
- Sharma BR, Joshi AS, Varthakavi PK, et al. Celiac autoimmunity in autoimmune thyroid disease is highly prevalent with a questionable impact. Indian J Endocrinol Metab 2016;20(1):97-100. [Full text]
- Roy A, Laszkowska M, Sundström J, et al. Prevalence of celiac disease in patients with autoimmune thyroid disease: a meta-analysis. Thyroid 2016;26(7):880-890. [Abstract]
- Drago S, El Asmar R, Di Pierro M, et al. Gliadin, zonulin and gut permeability: effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand J Gastroenterol 2006;41(4):408-419. [Abstract]
- Fasano A. Leaky gut and autoimmune diseases. Clin Rev Allergy Immunol 2012;42(1):71-78. [Abstract]
- Park JH, Jeong SY, Choi AJ, et al. Lipopolysaccharide directly stimulates Th17 differentiation in vitro modulating phosphorylation of RelB and NF-κB1. Immunol Lett 2015;165(1):10-19. [Abstract]
- Orlando A, Linsalata M, Notarnicola M, et al. Lactobacillus GG restoration of the gliadin induced epithelial barrier disruption: the role of cellular polyamines. BMC Microbiol 2014;14:19. [Full text]
- Anderson RC, Cookson AL, McNabb WC, et al. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol 2010;10:316. [Full text]
- Liu Z, Li C, Huang M, et al. Positive regulatory effects of perioperative probiotic treatments on postoperative liver complications after colorectal liver metastases surgery: a double-center and double-blind randomized clinical trial. BMC Gastroenterol 2015;15:34. [Full text]
- Skrovanek S, DiGuilio K, Bailey R, et al. Zinc and gastrointestinal disease. World J Gastrointest Pathophysiol 2014;5(4):496-513. [Full text]
- Finamore A, Massimi M, Conti Devirgiliis L, et al. Zinc deficiency induces membrane barrier damage and increases neutrophil transmigration in Caco-2 cells. J Nutr 2008;138(9):1664-1670. [Full text]
- Wang J, Lv S, Chen G, et al. Meta-analysis of the association between vitamin D and autoimmune thyroid disease. Nutrients 2015;7(4):2485-2498. [Full text]
- Kim D. Low vitamin D status is associated with hypothyroid Hashimoto's thyroiditis. Hormones (Athens). 2016;[Epub ahead of print]. [Full text]
- Arslan MS, Ekiz F, Deveci M, et al. The relationship between cytotoxin-associated gene A positive Helicobacter pylori infection and autoimmune thyroid disease. Endocr Res 2015;40(4):211-214. [Abstract]
- Radić M. Role of Helicobacter pylori infection in autoimmune systemic rheumatic diseases. World J Gastroenterol 2014;20(36):12839-12846. [Full text]
- Di Crescenzo V, D'Antonio A, Tonacchera M, et al. Human herpes virus associated with Hashimoto's thyroiditis. Infez Med 2013;21(3):224-228. [Full text]
- Draborg AH, Duus K, Houen G. Epstein-Barr virus in systemic autoimmune diseases. Clin Dev Immunol 2013;2013:535738. [Full text]
- Gaberšček S, Zaletel K, Schwetz V, et al. Mechanisms in endocrinology: thyroid and polycystic ovary syndrome. Eur J Endocrinol 2015;172(1):R9-21. [Abstract]
- Arduc A, Aycicek Dogan B, Bilmez S, et al. High prevalence of Hashimoto’s thyroiditis in patients with polycystic ovary syndrome: does the imbalance between estradiol and progesterone play a role? Endocr Res 2015;40(4):204-210. [Abstract]
- Jara LJ, Medina G, Saavedra MA, et al. Prolactin and autoimmunity. Clin Rev Allergy Immunol 2011;40(1):50-59. [Abstract]
- Nociti V, Frisullo G, Tartaglione T, et al. Multiple sclerosis attacks triggered by hyperprolactinemia. J Neurooncol 2010;98(3):407-409. [Full text]
- Legorreta-Haquet MV, Chávez-Rueda K, Chávez-Sánchez L, et al. Function of Treg cells decreased in patients with systemic lupus erythematosus due to the effect of prolactin. Medicine (Baltimore) 2016;95(5):e2384. [Full text]
- van Die MD, Burger HG, Teede HJ, et al. Vitex agnus-castus extracts for female reproductive disorders: a systematic review of clinical trials. Planta Med 2013;79(7):562-575. [Abstract]
- Yu Q, Wang JB. Subclinical hypothyroidism in PCOS: impact on presentation, insulin resistance, and cardiovascular risk. Biomed Res Int 2016;2016:2067087. [Full text]
- Tenazzani AD, Despini G, Santagni S, et al. Effects of a combination of alpha lipoic acid and myo-inositol on insulin dynamics in overweight/obese patients with PCOS. Endocrinol Metab Synd 2014;3:3. [Full text]
- Morgante G, Cappelli V, Di Sabatino A, et al. Polycystic ovary syndrome (PCOS) and hyperandrogenism: the role of a new natural association. Minerva Ginecol 2015;67(5):457-463. [Abstract]
- Mortimer RH. Thyroid function tests. Aust Prescr 2011;34:12-15. [Full text]
- The Australian Thyroid Foundation Ltd. Thyroid conditions. Viewed 6 Spetember 2016, https://www.thyroidfoundation.org.au/page/58/thyroid-conditions
- Act for Libraries. Difference between Hashimotos thyroiditis and Graves disease. Viewed 6 September, http://www.actforlibraries.org/difference-between-hashimotos-thyroiditis...
- Lab Tests Online. Thyroid antibodies, 2015. Viewed 6 September 2016, https://labtestsonline.org/understanding/analytes/thyroid-antibodies/tab...
DISCLAIMER:
The information provided on FX Medicine is for educational and informational purposes only. The information provided on this site is not, nor is it intended to be, a substitute for professional advice or care. Please seek the advice of a qualified health care professional in the event something you have read here raises questions or concerns regarding your health.