Female Reproductive Hormones and Immunity
by Georgia Marrion MHNut, BHSc (Comp Med), Adv Dip Health Sci (Nat).
Sexual dimorphism influences many aspects of health including immune function, responsivity and disease susceptibility[1,2]. Such sex-based differences are mediated by a complex interplay between genetic and hormonal factors particularly involving the X chromosome (which carries innate and adaptive immune genes) and steroid hormones[2]. Steroid hormones regulate immune system development, homeostasis, gene expression, signalling and immune cell functionality[2,3]. Consequently, hormonal fluctuations during the menstrual cycle and different life stages significantly influences immune function. This article reviews how the interplay between female steroid hormones and the immune system affects reproductive health.
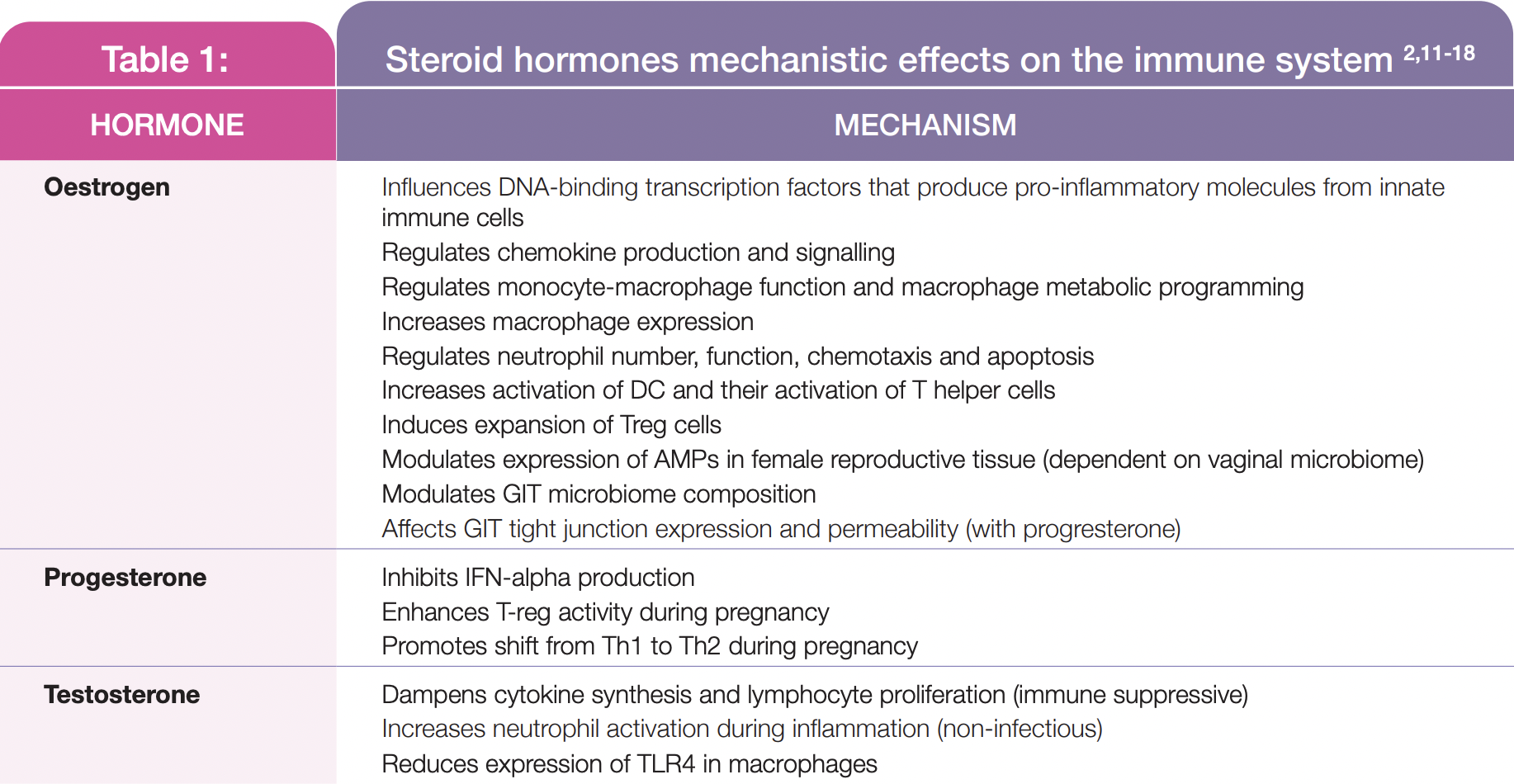
Oestrogens induce their biological activity through alpha and beta oestrogen receptors (ERalpha and ERbeta)[4-6]. Sex steroid hormones oestrogen (primarily 17beta oestradiol [E2]) and progesterone regulate the menstrual cycle, fertilisation, implantation and early embryonic development[7,8]. In premenopausal women, oestrogen synthesis from the developing ovarian follicles during the follicular phase increases until ovulation. Following ovulation, during the luteal phase, progesterone derived from the corpus luteum increases until the onset of menstruation[7]. During perimenopause, both oestrogen and progesterone levels progressively decline, with significant fluctuations in these hormones occurring in the transition to menopause[7]. Conversely, in pregnancy, oestrogen and progesterone levels increase throughout gestation[9]. Steroid hormones regulate immunity via a range of mechanisms (see Table 1) and, reciprocally, the immune system is closely involved in many reproductive processes in pregnant, pre- and post-menopausal women under the influence of the characteristic steroidal hormonal patterns that occur during these life stages.
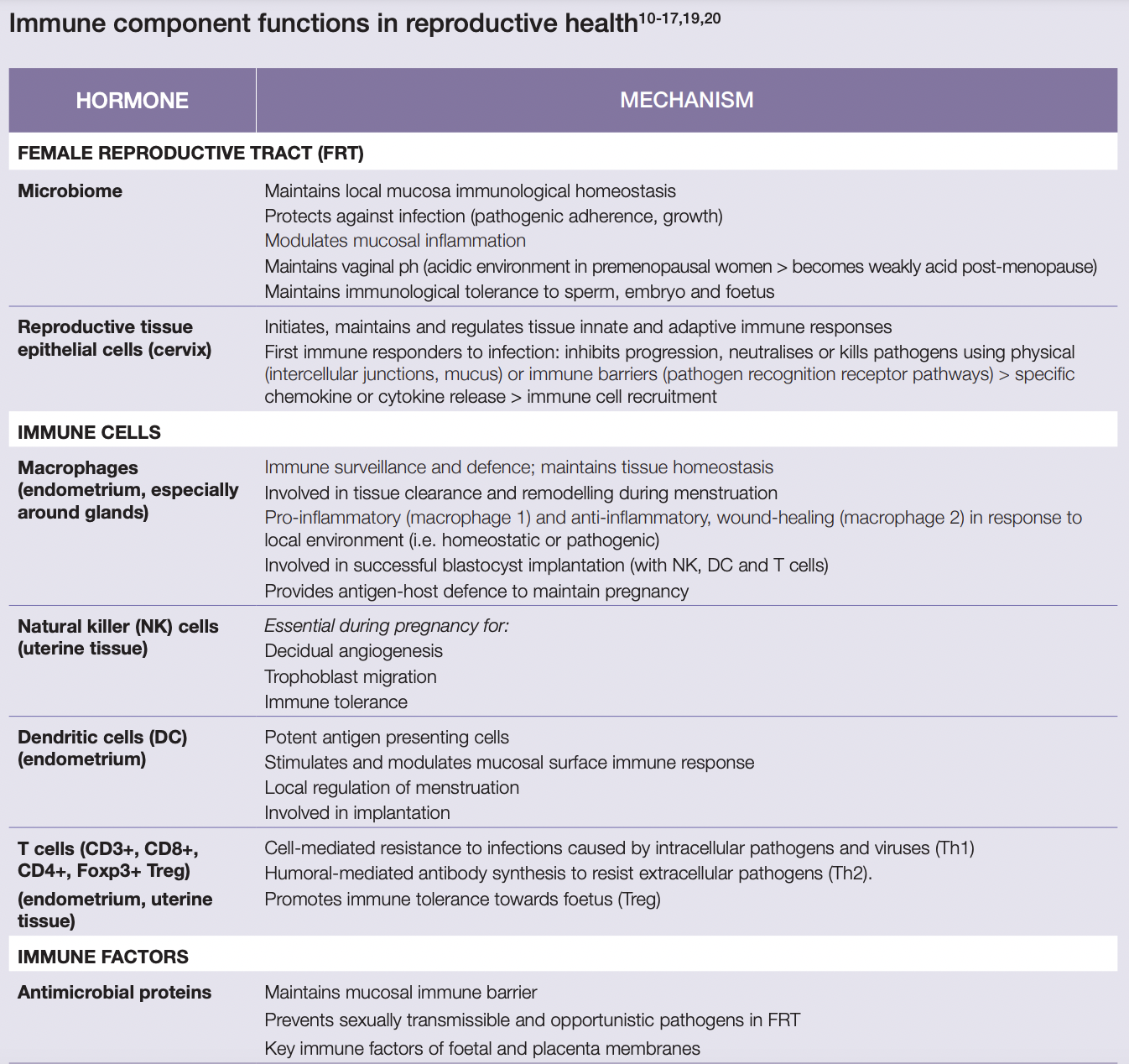
This involves complex interactions between cervical epithelial cells; female reproductive tract and gastrointestinal microbiota; and immune cells concentrated in the female reproductive tract (T cells, macrophages, neutrophils and mast cells, dendritic and natural killer cells)[10,11].
Menstrual cycle changes in immune system components
During the follicular phase, there is an increase in angiogenesis, tissue regeneration and AMP secretions; also, local immune cell concentrations are reduced and proinflammatory cytokine secretion from uterine epithelial cells are inhibited by elevated oestrogen levels[10,11]. High oestrogen also supports vaginal microbiome proliferation, with higher Lactobacilli concentrations through until ovulation[15,21].
At the onset of ovulation, angiogenesis, AMPs and epithelial barrier proteins are at their highest. Uterine endometrial cells, mediated by sex steroid hormones, also produce cytokines and chemokines which induce the recruitment of local immune cells (NK cells, neutrophils macrophages). Cervical and vaginal IgA, IgG, IL-6 and IL-8 concentrations are reduced to promote sperm cell survival[10,11,22].
During the luteal phase, when sex steroid hormones decline as menses approaches, these immune cell concentrations continue to increase as an inflammatory response is initiated. Local AMP synthesis increases, while local cytotoxic cell activity and neutrophil bactericidal activity decreases, to enhance the capacity for implantation. If a pregnancy does not occur, a local inflammatory response involving immune cells and cytokines causes subsequent tissue disintegration, resulting in the onset of menstruation when lower vaginal microbiota diversity is occurs[10,15,22,23].
If conception takes place, a complex communication ensues between many endometrial, foetal and placental tissue immune factors throughout the pregnancy. This enables blastocyst implantation and placental and foetal growth; it also promotes immune tolerance of the foetus’ hemiallogeneic tissue and protects it from infection, promoting an environment that supports pregnancy maintenance[2,17,18]. The increased systemic inflammatory environment and higher prevalence and risk of infections in peri- and post-menopausal women is associated with the impact of reduced oestrogen and progesterone levels on immune system functionality[2,10,13]. This includes reductions in immune cell concentrations and activity and immune factors (AMP, cervical mucous)[1,10,13]. Significant alterations in the vaginal microbiome occurs including decreased Lactobacilli diversity and abundance and reduced NK cell activity, mucosal, cell-mediated and humoral immunity[17,21].
This complex interplay between steroid hormones and many aspects of the immune system can become dysregulated, adversely affecting reproductive health in pre- and post-menopausal women and during pregnancy, including those listed in Table 2.
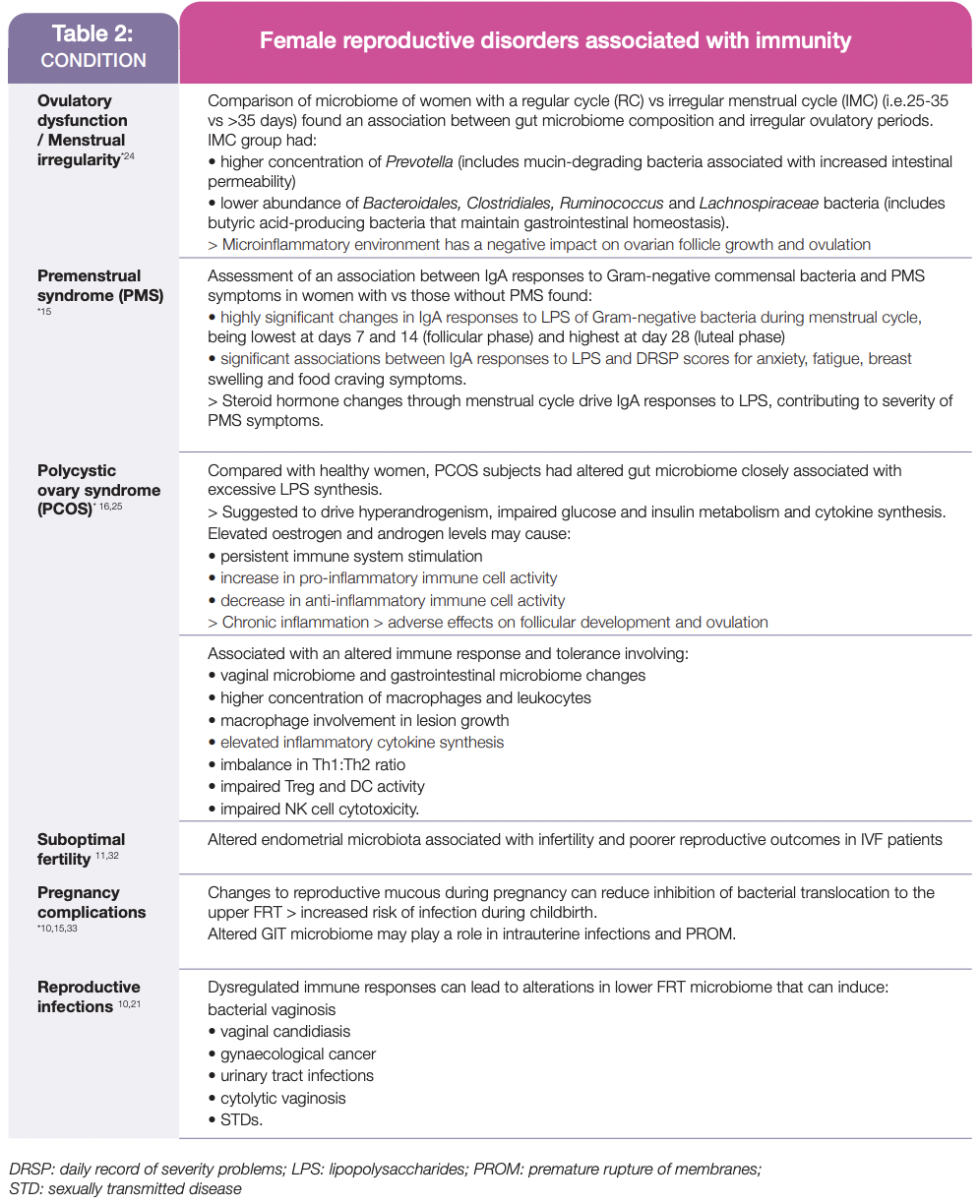
Overall, this review highlights that effective management of female reproductive health requires assessment of the bidirectional relationship between steroid hormones and immune system factors on the development, progression and severity of many conditions experienced by pregnant, pre- and post-menopausal women.
1. Taneja V. Sex hormones determine immune response. Front Immunol 2018; 9:1931.
2. Jaillon S, Berthenet K, Garlanda C. Sexual dimorphism in innate immunity. Clin Rev Allerg Immunol 2017;doi: 10.1007/s12016-017-8648-x.
3. Moulton VR. Sex hormones in acquired immunity and autoimmune disease. Front Immunol 2018;9:2279.
4. Guillaume M, Montagner A, Fontaine C, et al. Nuclear and membrane actions of estrogen receptor alpha: contribution to the regulation to energy and glucose homeostasis. Adv Exp Med Biol 2017;1043:401-426.
5. Ma W, Chen X, Cerne R, et al. Catechol estrogens stimulate insulin secretion in pancreatic beta-cells via activation of the transient receptor potential A1 (TRPA1) channel. J Biol Chem 2019;294 8):2935-2946.
6. Qian SW, Liu Y, Wang J, et al. BMP4 cross-talks with oestrogen/ERa signalling to regulate adiposity and glucose metabolism in females. EBioMedicine. 2016;11:91-100.
7. Trickey R. Women, hormones and the menstrual cycle. Trickey Enterprises Pty Ltd. 2011: Melbourne.
8. Barreto-Andrade JN, de Fatima LA, Campello RS, et al. Oestrogen receptor 1 (ESR1) enhances Sic2a4/GLUT4 expression by a SP1 cooperative mechanism. Int J Med Sci 2018;15(12):1320-1328.
9. Hechtman L. Advanced clinical naturopathic medicine. Elsevier: Sydney. 2020.
10. De Tomasi JB, Opata MM, Mova CN. Immunity in the cervix: interphase between immune and cervical epithelial cells. J Immunol Res 2019;2019: 7693183.
11. Agostinis C, Mangogna A, Bossi F, et al. Uterine immunity and microbiota: a shifting paradigm. Front Immunol 2019;10:2387.
12. Yarbrough VL, Winkle S, Herbst-Kralovetz MM. Antimicrobial peptides in the female reproductive tract: a critical component of the mucosal immune barrier with physiological and clinical implications. Hum Reprod Update 2015;21(3):353-77.
13. Trenti A, Tedesco S, Boscaro C, et al. Estrogen, angiogenesis, immunity and cell metabolism: solving the puzzle. Int J Mol Sci 2018;19(3):859.
14. Baker JM, Al-Nakkash L, Herbst-Kralovetz MM. Estrogen-gut microbiome axis: physiological and clinical implications. Maturitas 2017;103:45-53.
15. Roomruangwong C, Carvalho AF, Geffard M, Maes M. The menstrual cycle may not be limited to the endometrium but may also impact permeability. Acta Neuropsychiatrica 2019;31:294-304.
16. Zhou Z, Zhang L, Ding M, et al. Oestrogen decreases tight junction protein ZO-1 expression in human primary gut tissues. Clin Immunol 2017;183:174-180.
17. Villa P, Cipolla C, D’Ippolito S, et al. The interplay between immune system and microbiota in gynecological diseases: a narrative review. Eur Rev Med Pharmac 2020;24:5676-5690.
18. Giefing-Kroll C, Berger P, Lepperdinger G, Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections and response to vaccination. Aging Cell 2015;14(3):309-321.
19. Xu J, Bian G, Zheng M, et al. Fertility factors affect the vaginal microbiome in women of reproductive age. Am J Reprod Immunol 2020;83(4):e13220.
20. Dabee S, Passmore JS, Heffron R, Jaspan HB. The complex link between the female genital microbiota, genital infections and inflammation. Imm Infect 2021;doi: 10.1128/1A1.00487-20.
21. Gupta S, Kakkar V, Bushan I. Crosstalk between vaginal microbiome and female health: a review. Microb Pathogenesis 2019;136:103696.
22. Bradley F, Birse K, Hasselrot K, et al. The vaginal microbiome amplifies sex-hormone associated cyclic changes in cervicocavinal inflammation and epithelial barrier disruption. Am J Repro Immunol 2018;80(1):e12863.
23. Smirnova TG, Savachkina AY, Dolgushin II, et al. Changes in functional activity of neutrophils and monocytes isolated from the peripheral blood of women at different phases of the menstrual cycle. Bull Exp Biol Med 2018;166(2):222-224.
24. Sasaki H, Kawamura K, Kawamura T, et al. Distinctive subpopulations of the intestinal microbiota are present in women with unexplained chronic anovulation. Repro Biomed Online 2019;38(4):570-578.
25. Hu C, Pang B, Ma S, Hi H. Immunophenotypic profiles in polycystic ovary syndrome. Mediators Inflamm 2020;2020:5894768.
26. Perrotta AR, Borrelli GM, Matins CO, et al. The vaginal microbiome as a tool to predict rASM stage of disease in endometriosis: a pilot study. Repro Soc 2020;27(4):1064-1073.
27. Leonardi M, Hicks C, El-Asaad F, et al. Endometriosis and the microbiome: a systematic review. BJOG 2020;127(2):239-249.
28. Liu YY, Liu YK, Hu WT, et al. Elevated heme impairs macrophage phagocytosis in endometriosis. Reproduction 2019;158(3):257-266.
29. Lagana AS, Salmeri FM, Frangez HB, et al. Evaluation of M1 and M2 macrophages in ovarian endometriomas from women affected by endometriosis at different stages of the disease. Gynecol Endocrinol 2020;36(5):441-444.
30. Zhang T, De Caraolis C, Man GCW, Wang CC. The link between immunity, autoimmunity and endometriosis: a literature update. Autoimmune Rev 2018;17(10):945-955.
31. Vallve-Juanico J, Houshdaran S, Guidice LC. The endometrial immune environment of women with endometriosis. Hum Repr Update 2019;25(5):564-591.
32. Moreno I, Codoner FM, Vilella F, et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am J Obstet Gunecol 2016;215(6):684-703.
33. Edwards SM, Cunningham SA, Dunlop AL, Corwin EJ. The maternal gut microbiome during pregnancy. Am J Matern Child Nurs 2017;42(6):310-317.




