In the past, genetics were believed to play a leading role in the development of autoimmune illness, however emerging evidence now demonstrates that this genetic susceptibility is largely insufficient to determine disease onset.[1]
It is impossible to cover all important considerations for the treatment of autoimmune illness in a single piece, and therefore this article focuses primarily on some specific nutritional and herbal interventions which emerging evidence is suggesting to be useful.
Immune dysfunction
Numerous derangements in immune function are present in autoimmune disease. Certain key observations include suppressed regulatory T cell (T reg) and IL-10 release. As these play a fundamental role in immune tolerance, aberrant immune responses result. Subsequent elevations in Th17 and Th1 cells occur, combined with increased production/release of numerous inflammatory cytokines (e.g. IL-6, IL-17, IL-22 and TNF-alpha) and NFkB (a driver of inflammation). The increase in Th17 is what drives the release of cytokines IL-17 and IL-22 and these are often responsible for not only inflammation, but also tissue destruction.[2]
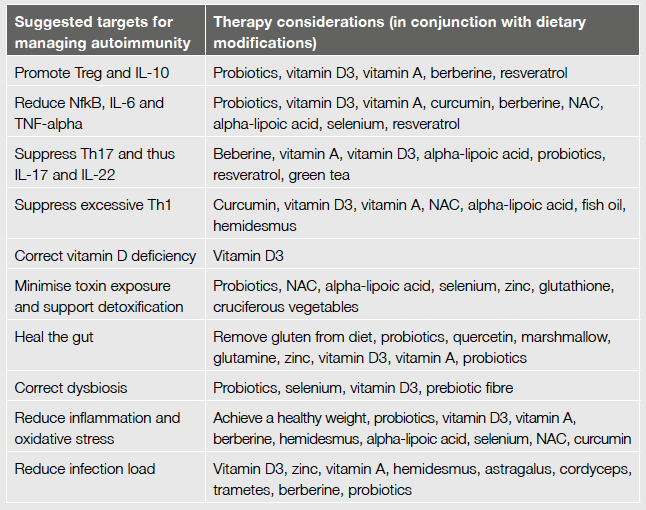
A suggested treatment for patients with autoimmune-related illness would thus include measures which promote T reg and IL-10 (to restore immune tolerance), and suppress inordinate Th17, Th1, IL-6, IL-17, IL-22, TNF-alpha and NFkB.
Contributors to autoimmune risk
- Genetic susceptibility
- T cell dysregulation (high Th17 and Th1, low T reg)
- Vitamin D deficiency Vitamin A deficiency
- Obesity
- Poor diet
- Oxidative and toxic stress (e.g. mercury)
- Infection load
- Intestinal barrier dysfunction
- Dysbiosis
Further considerations:
- Molecular mimicry
- Foetal microchimerism
- Adrenal fatigue - emotional stressors
Restoring immune tolerance
As mentioned, specific nutritional deficiencies are linked to immune imbalance and so too is dysbiosis and intestinal hyper-permeability. Toxicity, infection load and oxidative stress are also culprits. Therefore identification and treatment of these factors are an ideal place to start.
Vitamin D and the microbiota
T cells express vitamin D receptors,[3] and vitamin D favourably influences the immune system. It does this by promoting non-specific immune responses to infection (e.g. release of the antimicrobial peptide, cathelecidin), and also playing a role in the release of IL-10 and T reg.[4,5] Furthermore, vitamin D influences result in reduced release of Th17 and subsequently lower levels of IL-17 and other cytokines and immune cells commonly associated with an autoimmune response.[5]
Activation of the vitamin D receptor not only promotes IL-10 to suppress local and widespread inflammation, it also influences the composition of the gut microbiota.[3] Interestingly, fermentation of prebiotic fibres by commensal organisms and the resultant release of beneficial compounds are shown to increase the local expression of vitamin D receptors.
The absence of dysbiosis and consumption of a fibre-rich diet is therefore paramount in maximising the effectiveness of vitamin D, in addition to the well-known role these play in supporting immunological tolerance to the environment. This is noted as a significant finding for those with autoimmune inflammatory bowel diseases (ulcerative colitis, Crohn’s).
Furthermore, vitamin D is paramount for maintaining intestinal barrier integrity, necessary in prevention of the excessive immune activation and inflammation associated with autoimmunity. Lipopolysaccharide (LPS) endotoxin passage through a leaky gut barrier is a noted initiator of a Th17-driven response.[1,6] A compromised gut barrier also contributes to greater levels of overall systemic toxin load and oxidative stress, factors also associated with autoimmune disease.
A point to note here, is that via its ability to promote zonulin (a contributor to leaky gut), gluten may be a dietary antigen to consider for removal from the diet when restoring barrier integrity is the aim.[7]
Vitamin A
Vitamin A plays a somewhat similar role in maintaining immune balance and supporting intestinal integrity. It is essential in the production of secretory IgA (important at the epithelial surfaces for supporting immune tolerance), and its functions also include suppression of inflammatory IL-6, reduced Th17 and promotion of T reg.[5]
Antioxidants and detoxification
Maximising glutathione levels and function also appear to be useful in autoimmune states. Selenium is an essential component of the selenoprotein glutathione peroxidase, and has proven useful in autoimmune and inflammatory illness. Selenium status is also shown to impact microbial colonisation in the intestinal lumen and research supports selenium’s benefit in autoimmune illness, e.g. thyroiditis.
Alpha-lipoic acid (ALA) increases intercellular levels of glutathione by supporting the regeneration of L-cysteine, the rate limiting substrate in glutathione synthesis. ALA is also an antioxidant in its own right, supports toxic metal removal and regenerates other antioxidants including CoQ10. It has been shown to attenuate the inflammation initiated by endotoxin passage allowed by a leaky gut barrier.
Considering its lipophilic and hydrophilic nature, ALA is able to cross the blood brain barrier a feature which makes it useful not only in systemic, but also central nervous system-related illness. Animal and human studies (administering 1200mg ALA) have each supported the use of ALA in arresting inflammatory aspects of multiple sclerosis (MS).[8]
Certain toxic chemicals in the environment are noted to contribute significantly to autoimmune disease risk. Research published in February of this year in the United States found that in women of reproductive age (16-49yrs), exposure to methyl mercury (at low levels generally considered safe) was associated with subclinical autoimmunity (e.g. MS, systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA)). Furthermore the authors concluded that exposure to mercury “stood out as the main risk factor for autoimmunity”. The higher their levels of mercury, the higher the levels of autoantibodies.[9]
The necessity of maintaining intestinal integrity is important also for minimising heavy metal passage and supporting efficient toxin excretion. Furthermore, a healthy microbiota is useful in mitigating internal exposure to heavy metals, as these support barrier function and may sequester toxic metals, preventing their infiltration. As healthy microbial colonisation also promotes immune tolerance, the restoration and maintenance of the gut microbiota cannot be underestimated in autoimmune and inflammatory illness.
N-acetylcysteine (NAC) is noted to promote healthy glutathione synthesis for supporting metal removal, whilst also acting as as an antioxidant individually. Studies demonstrate NAC to be useful in protecting against metal-induced toxic organ damage. It also demonstrates anti-inflammatory benefits, and animal studies suggests its role in ameliorating induced models of autoimmune disease.[10,11] Significant oxidative stress and mitochondrial dysfunction are common features in conditions such as SLE and, considering NAC is shown to inhibit these,[12] consideration of its use as a therapy is warranted.
Infection load
The Epstein-Barr virus (EBV) is suspected to play a role in the pathogenesis of systemic autoimmune diseases. For example, SLE patients are seen to have abnormally high viral loads in peripheral blood mononuclear cells when compared to healthy controls (10-40 fold increase). Viral load is also associated with disease activity. It is possible that reactivation of EBV is associated with the development of SLE flares.[13]
The presence of unresolved infection contributes to persistent activation of the immune system, potentially depleting the antioxidant system (e.g. glutathione) which plays a role in the function of natural killer cells, but also supports detoxification and attenuates inflammation.
Supporting immune defences (without over-stimulation of the immune system) is therefore useful. Nutrients such a vitamins A, C and D, zinc, glutathione, and herbs such as hemidesmus, phellodendron (standardised to berberine) and astragalus, medicinal mushrooms (e.g. cordyceps and trametes) and probiotics all support defences via immunomodulatory mechanisms.
Herbal management
Hemidesmus is traditionally used in inflammatory and autoimmune conditions and is often the first herb that comes to mind when looking to calm autoimmune conditions such as RA. Limited human data is available on the clinical mechanisms of action displayed by hemisdemus, however research suggests antioxidant, anti-inflammatory, bacteriostatic, antibacterial, antifungal, antiviral and immune-modulating functions, all of which may benefit autoimmune patients. Further useful effects include pain relief, antiulcer, nephroprotective and anti tumour functions.[14,15]
Berberine, an active constituent found in a number of herbs (e.g. phellodendron), is receiving a great deal of attention in a variety of research fields. In preliminary studies, berberine has been shown to exert immunosuppressive and anti-inflammatory effects in several autoimmune diseases. It has been shown to inhibit the differentiation and function of Th1 and Th17 cells. Its effect on the Th17 response is observed to be mediated by a direct action on T cells as well as an indirect effect via dendritic cells. Berberine also reduces production of inflammatory IL-6 via inhibition of NFkB activity.[16,17]
Reduce fat mass
The western diet and lifestyle is contributing to increased risk of autoimmune disease. Many etiological studies in humans have shown a strong correlation between obesity and inflammatory autoimmune diseases.[18]
Obesity in itself is an inflammatory state, plus elevations in specific adipokines (released by adipocytes) such as leptin, are associated positively with the onset of specific autoimmune disease states (e.g. MS, RA, psoriasis, SLE and ulcerative colitis).[19,20]
Leptin acts as a cytokine mediator, influencing the release of other immune chemicals including pro-inflammatory cytokines and appears to be involved in T cell differentiation into Th17 cells.[21] Leptin is a beneficial chemical, required in sufficient amounts for healthy immune response to infection, however its release in excess is a concern.
The obesity epidemic may also be contributing to the vitamin D deficiency associated with higher risk of autoimmunity (amongst many other conditions). Fat mass sequesters vitamin D in the body, thus being obese contributes significantly to deficiency risk.
In conclusion, beneficial considerations for an autoimmune treatment strategy should include correction of nutritional deficiencies (e.g. vitamin D and A, selenium and zinc) and weight, management of dysbiosis, support for GIT integrity and detoxification, reduction of exposure to toxins and dietary antigens of concern, plus anti-inflammatory, antioxidant and anti-infection measures. Paying special attention to specific symptoms associated with the relevant autoimmune disease will also be important.
References
- Parks CG, Miller FW, Pollard KM, et al. Expert panel workshop consensus statement on the role of the environment in the development of autoimmune disease. Int J Mol Sci 2014;15(8):14269-14297. [Full text]
- Vojdani A, Lambert J. The Role of Th17 in neuroimmune disorders: target for CAM therapy. Part I. Evid Based Complement Alternat Med 2011;2011:927294. [Full text]
- Cantorna MT, McDaniel K, Bora S, et al. Vitamin D, immune regulation, the microbiota, and inflammatory bowel disease. Exp Biol Med (Maywood) 2014;239(11):1524-1530. [Abstract]
- Vojdani A, Lambert J, Kellermann G. The role of Th17 in neuroimmune disorders: a target for CAM therapy. Part III. Evid Based Complement Alternat Med 2011;2011:548086. [Full text]
- Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol 2008;8(9):685-698. [Full text]
- Vojdani A, Lambert J. The Role of Th17 in neuroimmune disorders: target for CAM therapy. Part II. Evid Based Complement Alternat Med 2011;2011:984965. [Full text]
- Drago S, El Asmar R, Di Pierro M, et al. Gliadin, zonulin and gut permeability: effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand J Gastroenterol 2006;41(4):408-419. [Abstract]
- Khalili M, Azimi A, Izadi V, et al. Does lipoic acid consumption affect the cytokine profile in multiple sclerosis patients: a double-blind, placebo-controlled, randomized clinical trial. Neuroimmunomodulation 2014;21(6):291-296. [Abstract]
- Somers EC, Ganser MA, Warren JS, et al. Mercury exposure and antinuclear antibodies among females of reproductive age in the United States: NHANES. Environ Health Perspect 2015;123(8):792-798. [Full text]
- Shimada K, Uzui H, Ueda T, et al. N-Acetylcysteine ameliorates experimental autoimmune myocarditis in rats via nitric oxide. J Cardiovasc Pharmacol Ther 2015;20(2):203-210. [Abstract]
- Wang G, Wang J, Ma H, et al. N-Acetylcysteine protects against trichloroethene-mediated autoimmunity by attenuating oxidative stress. Toxicol Appl Pharmacol 2013;273(1):189-195. [Abstract]
- Doherty E, Oaks Z, Perl A. Increased mitochondrial electron transport chain activity at complex I is regulated by N-acetylcysteine in lymphocytes of patients with systemic lupus erythematosus. Antioxid Redox Signal 2014;21(1):56-65. [Full text]
- Draborg AH, Duus K, Houen G. Epstein-Barr virus in systemic autoimmune diseases. Clin Dev Immunol 2013;2013:535738. [Full text]
- Das S, Bisht SS. The bioactive and therapeutic potential of Hemidesmus indicus R. Br. (Indian Sarsaparilla) root. Phytother Res 2013;27(6):791-801. [Abstract]
- George S, Tushar KV, Unnikrishnan KP, et al. Hemidesmus indicus (L.) R. Br. A review. J Plant Sc 2008;3(2):146-156. [PDF]
- Yang Y, Qi J, Wang Q, et al. Berberine suppresses Th17 and dendritic cell responses. Invest Ophthalmol Vis Sci 2013;54(4):2516-2522. [Full text]
- Qin X, Guo BT, Wan B, et al. Regulation of Th1 and Th17 cell differentiation and amelioration of experimental autoimmune encephalomyelitis by natural product compound berberine. J Immunol 2010;185(3):1855-1863. [Full text]
- Arai S, Miyazaki T. Impacts of the apoptosis inhibitor of macrophage (AIM) on obesity-associated inflammatory diseases. Semin Immunopathol 2014;36(1):3-12. [Full text]
- Hasenkrug KJ. The leptin connection: regulatory T cells and autoimmunity. Immunity 2007;26(2):143-145. [Full text]
- Tian G, Liang JN, Wang ZY, et al. Emerging role of leptin in rheumatoid arthritis. Clin Exp Immunol 2014;177(3):557-570. [Full text]
- Reis BS, Lee K, Fanok MH, et al. Leptin receptor signaling in T cells is required for Th17 differentiation. J Immunol 2015;194(11):5253-5260. [Abstract]
DISCLAIMER:
The information provided on FX Medicine is for educational and informational purposes only. The information provided on this site is not, nor is it intended to be, a substitute for professional advice or care. Please seek the advice of a qualified health care professional in the event something you have read here raises questions or concerns regarding your health.