Hashimoto’s thyroiditis (HT) is the most prevalent autoimmune thyroid disorder (AITD) globally, with a prevalence of 17.5% in women and 6% in men.
Characterised by loss of self-tolerance by the immune system, with T-cell attack and subsequent lymphocytic infiltration and destruction of the follicular cells by anti-thyroid antibodies, HT leads to fibrosis, atrophy, and loss of function of the thyroid gland.
Signs & symptoms
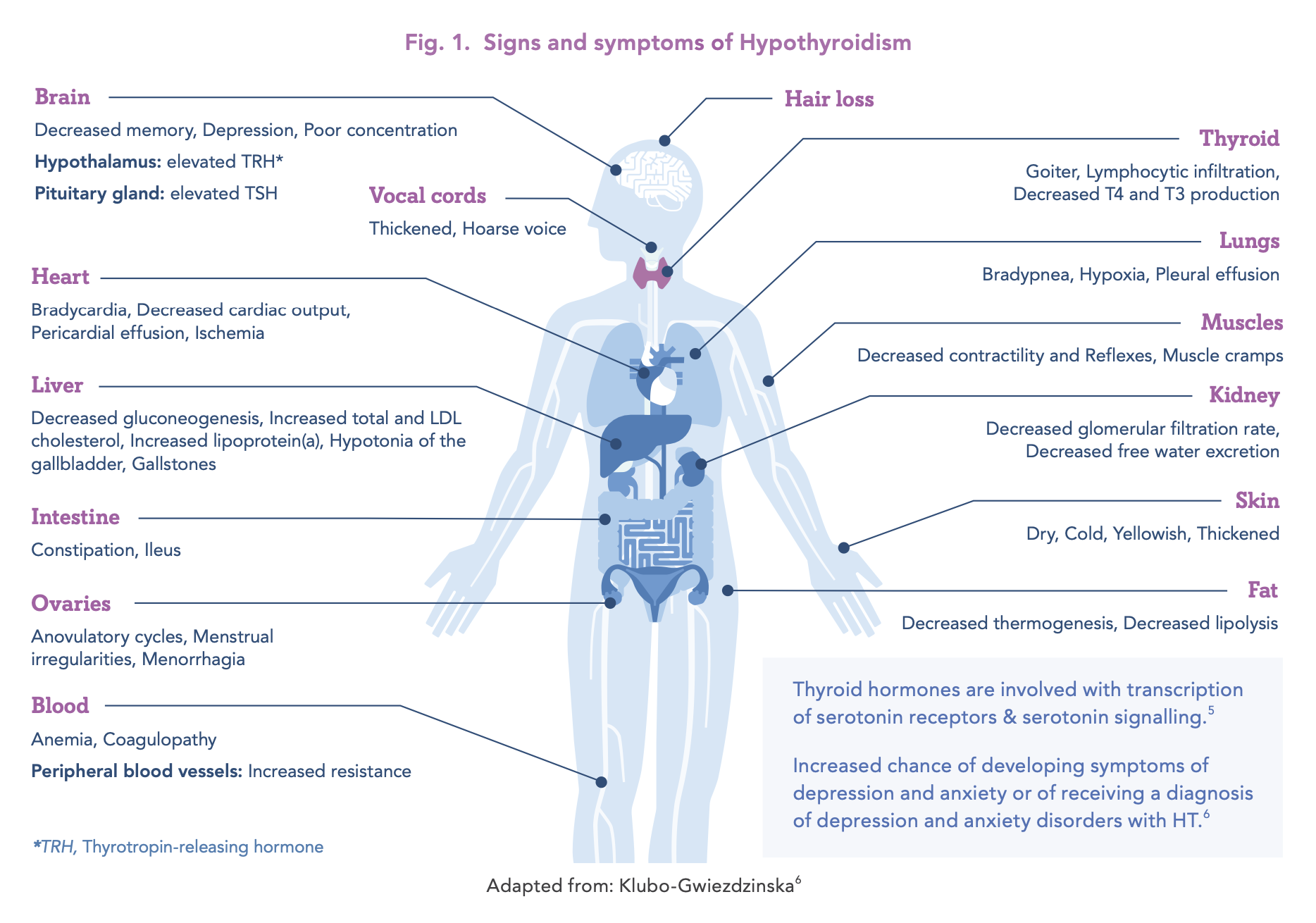
Signs and symptoms3,4 of HT may be mild and non-specific, particularly in the early stages of disease (see Figure 1). They include but are not limited to:
- Fatigue
- Weight gain
- Low mood
- Anxiety
- Cold intolerance
- Dry, scaly skin
- Poor concentration
- High cholesterol
- Bradycardia
- Sleep disturbances
Diagnosis
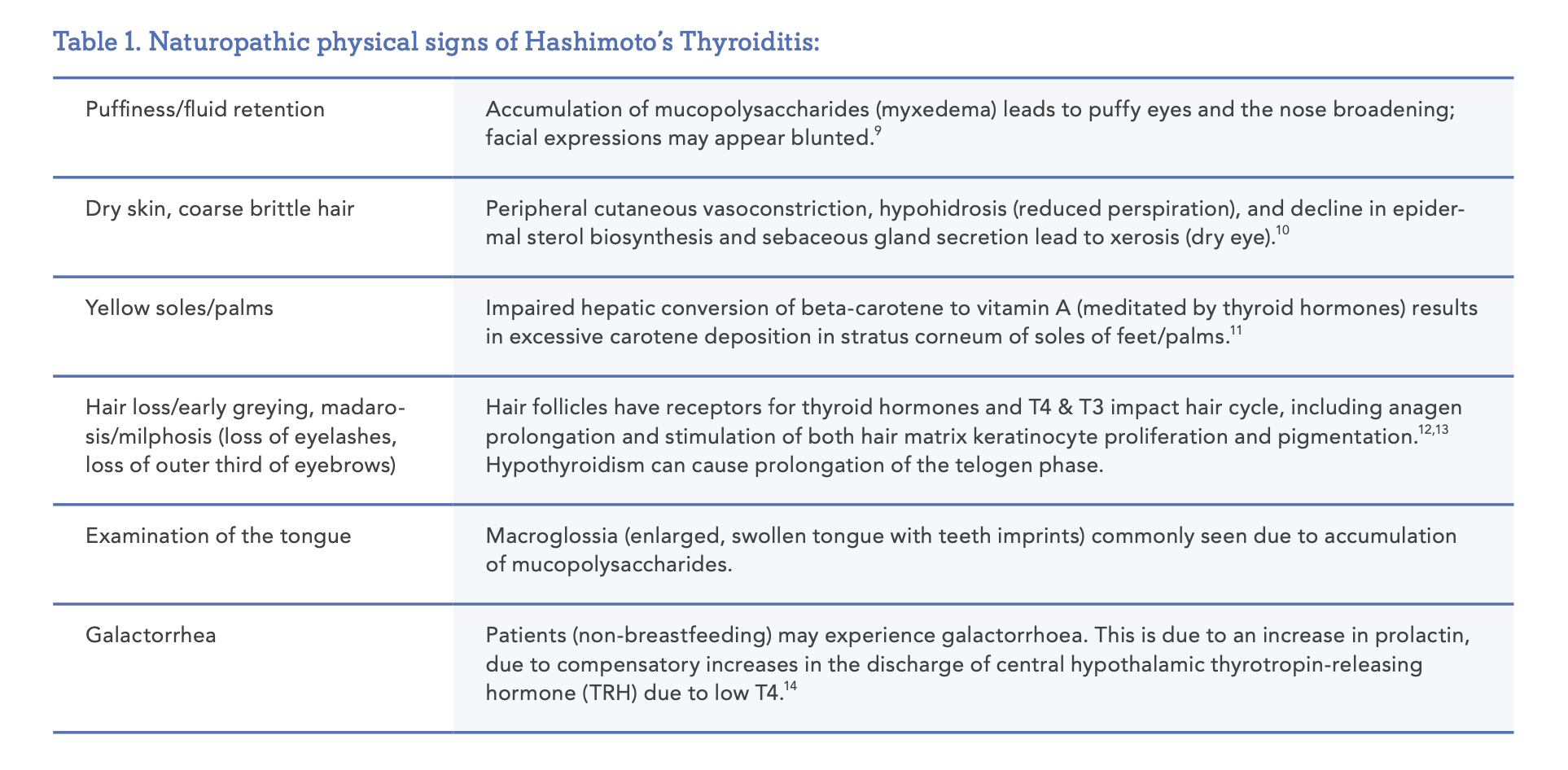
Diagnosis of HT includes the presence of circulating antibodies against the thyroid including anti-thyroid peroxidase (Anti-TPO) and anti-thyroglobulin antibodies (TG-Abs) with or without symptoms of hypothyroidism. It is important to note that a small percentage of individuals will present with clinical symptoms, positive ultrasound but seronegative antibodies.7,8 Physical signs of Hashimoto’s Thyroiditis are listed in Table 1.
Causes
HT is believed to be triggered by a complex interplay between genetic and environmental factors.
Genetics15
Polymorphisms in:
- Human leukocyte antigens (HLA) I & II
- Immunoregulatory genes
- Thyroid-specific genes e.g., thyroglobulin (TG)
- Genes associated with antibody synthesis (e.g. BACH2)
Environmental factors16
- Age: occurs at any age, however, peaks in 30-50 yr females
- Environmental toxins e.g., phthalates and flame retardants
- Presence of other autoimmune diseases increases risk
- Infections
- Sex steroids
Sex steroids
HT shows sex-specific and age-dependent incidences with females showing higher susceptibility than males hypothesised to be due to activation of genes on the X-chromosome.17 HT is commonly diagnosed in pregnancy,18 post-partum and perimenopause19 triggered by the complex interplay occurring between the immune system and sex hormones.
Practitioners should therefore be mindful to investigate the sex hormones and the menstrual cycle of a patient to ascertain whether these are a trigger for AIT dysfunction.
Stress & burnout
Sustained stress and trauma have been implicated in the pathogenesis of HT.20,21 It is believed that exposure to stressors result in activation of the hypothalamic-pituitary-adrenal-thyroid axis22 as well as redirection of the immune response from Th1 to Th2 dominance contributing to the development of HT. Prolonged psychological stress also impairs intestinal barrier function, driving dysbiosis and potential further autoimmune disease.23
Supplementation
Iodine
Iodine is necessary for optimal functioning of the thyroid gland including the manufacture of thyroid hormones. Excessive supplementation with iodine may damage thyrocytes via excess inflammation and oxidative stress induced by high amounts of iodine. Excess iodine can also induce autoimmunity, promoting the production of antibodies that function to damage and destroy thyroid hormones.24,25
Daily intake of iodine should remain at the RDI 150 mcg/day, increasing to 220 mcg/day in pregnancy and 270 mcg/day while breast-feeding.
Selenium
Selenium plays an important role in management of HT via the role of selenoproteins which are involved in iodothyronine deiodinase (ID), glutathione peroxidase (GSH-Px), and thioredoxin reductase (TR); supplementation has been shown to significantly decrease circulating TPO- Abs and TG-Abs in individuals with HT as well as decrease fT4/fT3 ratio, oxidative stress and inflammation.26,27
Optimal dosage appears to be 200 mcg/day for 3-6 months.28
Myo-inositol
Myo-inositol assists with biosynthesis of thyroid hormones. Supplementation has been shown to decrease thyroid antibodies and reduce pro-inflammatory chemokines.29 Combining myo-inositol and selenium appears to be beneficial.
In a randomised controlled trial involving 168 individuals with HT, supplementation with a combination of myo-inositol (600 mg/day) plus selenium (83 mcg/day) taken over 6 months was more effective in improving levels of TSH and decreasing TPO-Ab and Tg-Ab compared to using selenium (83 mcg/day) alone.30
Iron
Iron is required for the synthesis of thyroid hormones. Deficiency blocks the activity of thyroid peroxidase preventing synthesis of thyroid hormone and reduces conversion of T4 to T3.31 It is important that iron deficiency in HT may not always be due to dietary deficit; lack of thyroid hormone itself may be a driver of anaemia since thyroid hormones stimulate erythropoiesis by acting directly on bone marrow.32 In this situation, increasing levels of thyroid hormone will assist with improving iron status more successfully than just trying to supplement with iron alone. Where the anaemia is not responsive to supplementation (resistant anaemia) this may be due to anaemia of chronic disease (HT) or because of another co-existing condition such as coeliac disease that may be impairing absorption.
Optimal dosage of iron is dependant on deficiency and nutritional status.
Vitamin D
Low levels of vitamin D are associated with increased risk of HT. Meta-analysis reveal that vitamin D supplementation may reduce TGAb and TPOAb titres in individuals with HT.33 Beneficial effects of vitamin D supplementation appear to be increased if individuals received vitamin D3 and the duration of treatment was >3 months.34 The actions of vitamin D appear to be multifaceted but are hypothesised to involve immune modulation as well as its ability to reduce inflammation. Polymorphisms of the VDR gene have been implicated with increased risk of HT and may subsequently decrease vitamin D activity.35 This may explain why some individuals do not respond to vitamin D supplementation.
Research suggests up to 7000 IU/day of vitamin D to assist with modulating the immune system (depending on deficiency status).41,42
Zinc
Zinc is needed for the synthesis of TRH & TSH and functions as a co-factor for the deiodinases 1 & 2 assisting with conversion o fT4 to T3.36 Zinc is also required for expression of thyroid transcriptase factor 2 and subsequent gene expression of thyroglobulin and thyro-peroxidase.
Optimal dosage of zinc is dependant on deficiency and nutritional status.
Nutrition
Gluten free
A gluten free diet has been shown to assist with reducing serum titres of TPO-Ab and Tg-Ab in women with HT over a 6-month period.37,38 Consumption of gluten is associated with IP; Intestinal hyperpermeability is associated with auto-immune processes and the development of HT by promoting bacterial translocation and subsequent inflammation.
Autoimmune Protocol (AIP)
While the AIP diet is commonly recommended as a dietary strategy for the management of HT, it has not been shown to beneficially impact thyroid function in clinical studies. In a small study involving 17 women implementing the AIP diet, no statistically significant changes were noted in TSH, FT, FT3 or thyroid antibodies, however hs-CRP - a measure of inflammation - decreased significantly by 29% at the end of the intervention, suggesting it may be useful to decrease systemic inflammation in this population.39
Stress management
An 8-week RCT involving 60 women with HT observed a statistically significant decrease in TG-Abs as well as an improvement in mood (anxiety, depression) and perceived stress compared to the control group. Individuals in the intervention group were provided 8 weekly sessions of lifestyle modification and stress management, including diaphragmatic breathing, cognitive reconstruction, guided imagery, and diet adjustments compared to standard care.40 Changes in anti-TPO Abs and TSH were not statistically significant between the groups. The improvement in mood, stress, and decrease in TG-Abs highlights the efficacy of stress management as a tool for the management of HT.

Download a PDF of this article
References
1. Hu X et al. Global prevalence and epidemiological trends of Hashimoto’s thyroiditis in adults: A systematic review and meta-analysis. Front Public Health. 2022 Oct 13;10:1020709
2. Zhang QY, et al. Lymphocyte infiltration and thyrocyte destruction are driven by stromal and immune cell components in Hashimoto’s thyroiditis. Nat Commun. 2022 Feb 9;13(1):775.
3. Rotondi M et al. Serum-negative autoimmune thyroiditis: what’s in a name? J Endocrinol Invest 2014 37:589–591
4. Croce L Compared with classic Hashimoto’s thyroiditis, chronic autoimmune serum-negative thyroiditis requires a lower substitution dose of L-thyroxine to correct hypothyroidism. J Endocrinol Invest 2020 43:1631–1636
5. Weetman AP. An update on the pathogenesis of Hashimoto’s thyroiditis. J Endocrinol Invest. 2021 May;44(5):883-890
6. Klubo-Gwiezdzinska J, Wartofsky L. Hashimoto thyroiditis: an evidence-based guide to etiology, diagnosis and treatment. Pol Arch Intern Med. 2022 Mar 30;132(3):16222
7. Calcaterra V et al. Gender Differences at the Onset of Autoimmune Thyroid Diseases in Children and Adolescents. Front Endocrinol (Lausanne). 2020 Apr 17;11:229
8. Świątkowska-Stodulska R, et al. Endocrine Autoimmunity in Pregnancy. Front Immunol. 2022 Jun 29;13:907561
9. Stuenkel CA. Subclinical thyroid disorders. Menopause. 2015 Feb;22(2):231-3.
10. Jung SJ, et al. Posttraumatic stress disorder and incidence of thyroid dysfunction in women. Psychol Med. 2019 Nov;49(15):2551- 2560.
11. Jonsdottir IH et al. Mechanisms in Endocrinology: Endocrine and immunological aspects of burnout: a narrative review. Eur J Endocrinol. 2019 Mar 1;180(3):R147-R158
12. Fischer S et al. Effects of acute psychosocial stress on the hypothalamic-pituitary-thyroid (HPT) axis in healthy women. Psychoneuroendocrinology. 2019 Dec;110:104438.
13. Ilchmann-Diounou H, Menard S. Psychological Stress, Intestinal Barrier Dysfunctions, and Autoimmune Disorders: An Overview. Front Immunol. 2020 Aug 25;11:1823.
14. Patil N, et al. Hypothyroidism. [Updated 2022 Aug 8]. In: Stat-Pearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-Available from: https://www.ncbi.nlm.nih.gov/books/ NBK519536/
15. Klubo-Gwiezdzinska J, Wartofsky L. Hashimoto thyroiditis: an evidence-based guide to etiology, diagnosis and treatment. Pol Arch Intern Med. 2022 Mar 30;132(3):16222.
16. Touma KTB, et al. Liothyronine for Depression: A Review and Guidance for Safety Monitoring. Innov Clin Neurosci. 2017 Apr 1;14(3-4):24-29
17. Siegmann EM et al. Association of Depression and Anxiety Disorders With Autoimmune Thyroiditis: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2018 Jun 1;75(6):577-584.
18. Lause M, et al. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017 Oct;6(4):300-312.
19. Takir M, et al. Skin findings in autoimmune and nonautoimmune thyroid disease with respect to thyroid functional status and healthy controls. Turk J Med Sci. 2017 Jun 12;47(3):764-770.
20. Klubo-Gwiezdzinska J, Wartofsky L. Hashimoto thyroiditis: an evidence-based guide to etiology, diagnosis and treatment. Pol Arch Intern Med. 2022 Mar 30;132(3):16222
21. van Beek N et al. Thyroid hormones directly alter human hair follicle functions: anagen prolongation and stimulation of both hair matrix keratinocyte proliferation and hair pigmentation. J Clin Endocrinol Metab. 2008 Nov;93(11):4381-8.
22. Gharaei Nejad K et al. Dermoscopic Findings of Alopecia in Patients with Hypothyroidism. Int J Endocrinol Metab. 2022 Oct 11;20(4):e128938.
23. Koyyada A, Orsu P. Role of hypothyroidism and associated pathways in pregnancy and infertility: Clinical insights. Tzu Chi Med J. 2020 Apr 10;32(4):312-317.
24. Liu J, Mao C, Dong L, Kang P, Ding C, Zheng T, Wang X, Xiao Y. Excessive Iodine Promotes Pyroptosis of Thyroid Follicular Epithelial Cells in Hashimoto’s Thyroiditis Through the ROS-NF-kB-NLRP3 Pathway. Front Endocrinol (Lausanne). 2019 Nov 20;10:778. doi: 10.3389/fendo.2019.00778. PMID: 31824415; PMCID: PMC6880659.
25. Mikulska AA, et al. Metabolic Characteristics of Hashimoto’s Thyroiditis Patients and the Role of Microelements and Diet in the Disease Management-An Overview. Int J Mol Sci. 2022 Jun 13;23(12):6580.
26. Zuo Y, et al. The correlation between selenium levels and autoimmune thyroid disease: a systematic review and meta-analysis. Ann Palliat Med. 2021 Apr;10(4):4398-4408.
27. Wang LF et al. The effects of selenium supplementation on antibody titres in patients with Hashimoto’s thyroiditis. Endokrynol Pol. 2021;72(6):666-667.
28. Hu Y.et al. Effect of selenium on thyroid autoimmunity and regulatory T cells in patients with Hashimoto’s thyroiditis: A prospective randomized-controlled trial. Clin. Transl. Sci. 2021;14:1390–1402.
29. Paparo SR, et al. Myoinositol in Autoimmune Thyroiditis. Front Endocrinol (Lausanne). 2022 Jun 28;13:930756
30. Nordio M, Basciani S. Myo-inositol plus selenium supplementation restores euthyroid state in Hashimoto’s patients with subclinical hypothyroidism. Eur Rev Med Pharmacol Sci. 2017 Jun;21(2 Suppl):51-59.
31. Soliman AT et al. Chronic anemia and thyroid function. Acta Biomed. 2017 Apr 28;88(1):119-127.
32. Hernik A, et al. The hepcidin concentration decreases in hypothyroid patients with Hashimoto’s thyroiditis following restoration of euthyroidism. Sci Rep. 2019 Nov 7;9(1):16222
33. Taheriniya S, et al. Vitamin D and thyroid disorders: a systematic review and Meta-analysis of observational studies. BMC Endocr Disord. 2021 Aug 21;21(1):171.
34. Zhang J, et al. Effects of vitamin D on thyroid autoimmunity markers in Hashimoto’s thyroiditis: systematic review and meta-analysis. J Int Med Res. 2021 Dec;49(12):3000605211060675.
35. Feng M, et al. Polymorphisms in the vitamin D receptor gene and risk of autoimmune thyroid diseases: a meta-analysis. Endocrine. 2013 Apr;43(2):318-26.
36. Beserra JB et al. Relation Between Zinc and Thyroid Hormones in Humans: a Systematic Review. Biol Trace Elem Res. 2021 Nov;199(11):4092-4100.
37. Krysiak R, et al. The Effect of Gluten-Free Diet on Thyroid Autoimmunity in Drug-Naïve Women with Hashimoto’s Thyroiditis: A Pilot Study. Exp Clin Endocrinol Diabetes. 2019 Jul;127(7):417-422.
38. Ihnatowicz P, et al. The importance of gluten exclusion in the management of Hashimoto’s thyroiditis. Ann Agric Environ Med. 2021 Dec 29;28(4):558-568.
39. Abbott RD, et al. Efficacy of the Autoimmune Protocol Diet as Part of a Multi-disciplinary, Supported Lifestyle Intervention for Hashimoto’s Thyroiditis. Cureus. 2019 Apr 27;11(4):e4556. 40 Markomanolaki ZS, et al. Stress Management in Women with Hashimoto’s thyroiditis: A Randomized Controlled Trial. J Mol Biochem. 2019;8(1):3-12.
40. Markomanolaki ZS, et al. Stress Management in Women with Hashimoto’s thyroiditis: A Randomized Controlled Trial. J Mol Biochem. 2019;8(1):3-12.
41. Chahardoli R, et al. Can Supplementation with Vitamin D Modify Thyroid Autoantibodies (Anti-TPO Ab, Anti-Tg Ab) and Thyroid Profile (T3, T4, TSH) in Hashimoto’s Thyroiditis? A Double Blind, Randomized Clinical Trial. Horm Metab Res. 2019;51(5):296-301.
42. Robat-Jazi B, et al. The Impact of Vitamin D Supplementation on the IFNγ-IP10 Axis in Women with Hashimoto’s Thyroiditis Treated with Levothyroxine: A Double-blind Randomized Placebo-controlled Trial. Iran J Allergy Asthma Immunol. 2022;21(4):407-417.



