What is PEA?
Palmitoylethanolamide (PEA) is a bioactive lipid mediator that belongs to the N-acetylanolamine class of phospholipids. It was initially discovered in egg yolk, soybean, and peanut oil. In animal cells, PEA is synthesised from palmitic acid, a fatty acid present in foods such as palm oil, meats, cheeses, butter, and other dairy products.1-3 PEA is widely distributed around the body, appearing in the adrenal glands, diaphragm, spleen, kidney, testis, lung, liver, heart, plasma, erythrocytes, and retina. It penetrates the blood-brain barrier, primarily accumulating in the hypothalamus, pituitary, brain stem, cerebellum, and brain cortex.2
PEA and chronic inflammation
During pathological states, PEA levels are often altered. PEA is believed to be produced to help restore homeostasis after cellular injury and is usually upregulated under disease conditions.1 In the brain, PEA is produced as required by neurons, microglia, and astrocytes, thus playing a pleiotropic and prohomeostatic role. When faced with pro-inflammatory external stressors, PEA exerts a local anti-injury role by down-regulating mast cell activation and protecting neurons from excitotoxicity.2,3
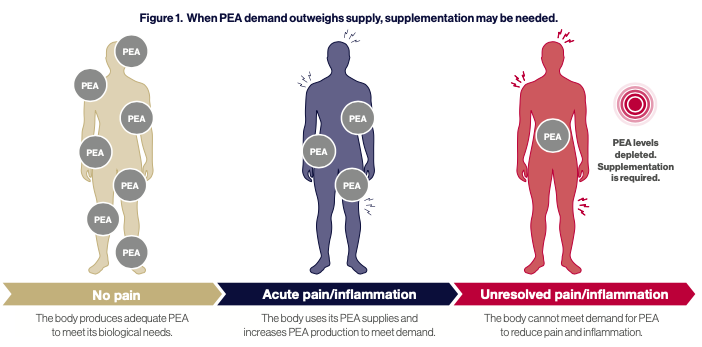
However, during chronic inflammation, tissue concentrations of endogenous PEA decrease due to reduced production and increased degradation. This makes endogenous PEA production inadequate to restore homeostasis. Therefore, supplementation with PEA presents a viable option to top up endogenous levels and restore bodily homeostasis.1 (See Figure. 1)
PEA mechanisms of action
PEA has anti-inflammatory, analgesic, anticonvulsant, antimicrobial, antipyretic, antiepileptic, immunomodulatory and neuroprotective activities. It exerts its analgesic and antiinflammatory effects primarily by activating the peroxisome proliferator-activated receptor alpha (PPAR)-α. Activation of the PPAR-α receptor initiates a range of actions that suppress pain and inflammatory signals, including inhibiting the release of pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6 and tumour necrosis factor-α. PEA also down-regulates mast-cell degranulation and regulates microglial activity. Moreover, even though PEA has a low affinity for the cannabinoid receptors CB1 and CB2 and, therefore, cannot be considered a classic endocannabinoid, it is an endogenous endocannabinoid-like compound that targets similar pathways to cannabinoids.1, 2, 4

Potential therapeutic effects of PEA
Because of PEA’s multiple mechanisms of action, it potentially has many therapeutic benefits, such as a treatment for allergic reactions, influenza, common cold, chronic pain, and joint pain.1 Currently, it is commonly used for the treatment of different types of neuropathic pain, such as fibromyalgia, osteoarthritis, and carpal tunnel syndrome.5-7 It may also reduce post-operative pain, enhance muscle recovery following exercise, and reduce the prevalence of migraines.7-9
Bioavailability of PEA
With its lipid structure and large particle sizes, PEA has poor solubility and bioavailability.4 To overcome these problems, PEA has been micronised and ultra-micronised, which has enhanced its absorption and bioavailability.
Using a novel crystalline dispersion technology (LipiSperse®), where a combination of surfactants, polar lipids and solvents are used to increase the wettability of lipophilic substances in aqueous environments, the increased absorption of PEA has also been achieved. Compared to non-micronised PEA, the administration of this unique PEA product known as Levagen®+ was associated with faster absorption and 1.75 times greater PEA plasma concentrations.17 This enhanced absorption is essential as lower dosages may be used to achieve therapeutic benefits.
Administration and dosing
The doses of PEA used in clinical trials vary but generally range from 300 mg to 600 mg daily. Typically, PEA is administered for 4 to 8 weeks, although if used as a neuroprotective agent, it may require administration for more extended periods.1, 3, 10
In sum, PEA is an exciting ingredient that can potentially have therapeutic benefits for a range of conditions. The bulk of evidence supports its pain-relieving properties, although it has promise as a natural agent to support mood, reduce neuroinflammation, and provide neuroprotection.
Download a pdf copy of this article
References
1 Clayton, P., et al., Palmitoylethanolamide: A Natural Compound for Health Management. Int J Mol Sci, 2021. 22(10).
2 Clayton, P., et al., Palmitoylethanolamide: A Potential Alternative to Cannabidiol. J Diet Suppl, 2023. 20(3): p. 505-530.
3 Landolfo, E., et al., Effects of Palmitoylethanolamide on Neurodegenerative Diseases: A Review from Rodents to Humans. Biomolecules, 2022. 12(5).
4 Petrosino, S. and V. Di Marzo, The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br J Pharmacol, 2017. 174(11): p. 1349-1365.
5 Lang-Illievich, K., et al., Palmitoylethanolamide in the Treatment of Chronic Pain: A Systematic Review and Meta-Analysis of Double-Blind Randomized Controlled Trials. Nutrients, 2023. 15(6).
6 Lang-Illievich, K., et al., The Effect of Palmitoylethanolamide on Pain Intensity, Central and Peripheral Sensitization, and Pain Modulation in Healthy Volunteers-A Randomized, Double-Blinded, Placebo-Controlled Crossover Trial. Nutrients, 2022. 14(19).
7 Gabrielsson, L., S. Mattsson, and C.J. Fowler, Palmitoylethanolamide for the treatment of pain: pharmacokinetics, safety and efficacy. Br J Clin Pharmacol, 2016. 82(4): p. 932-42.
8 Chirchiglia, D., et al., Effects of Add-On Ultramicronized N-Palmitol Ethanol Amide in Patients Suffering of Migraine With Aura: A Pilot Study. Front Neurol, 2018. 9: p. 674.
9 Mallard, A., et al., The Effect of Orally Dosed Levagen+ (palmitoylethanolamide) on Exercise Recovery in Healthy Males-A Double-Blind, Randomized, Placebo-Controlled Study. Nutrients, 2020. 12(3).
10 Colizzi, M., et al., Therapeutic effect of palmitoylethanolamide in cognitive decline: A systematic review and preliminary meta- analysis of preclinical and clinical evidence. Front Psychiatry, 2022. 13: p. 1038122.
11 Topuz, R.D., Y. Gorgulu, and M. Kyazim Uluturk, Could serum endocannabinoid and N-acylethanolamine levels be important in bipolar disorder? World J Biol Psychiatry, 2023. 24(4): p. 314-320.
12 Hauer, D., et al., Plasma concentrations of endocannabinoids and related primary fatty acid amides in patients with post- traumatic stress disorder. PLoS One, 2013. 8(5): p. e62741.
13 Ghazizadeh-Hashemi, M., et al., Palmitoylethanolamide as adjunctive therapy in major depressive disorder: A double-blind, randomized and placebo-controlled trial. J Affect Disord, 2018. 232: p. 127-133.
14 Salehi, A., et al., Adjuvant palmitoylethanolamide therapy with risperidone improves negative symptoms in patients with schizophrenia: A randomized, double-blinded, placebo-controlled trial. Psychiatry Res, 2022. 316: p. 114737.
15 Abedini, T., et al., Efficacy and safety of palmitoylethanolamide as an adjunctive treatment for acute mania: A randomized, double-blind, placebo-controlled trial. Psychiatry Clin Neurosci, 2022. 76(10): p. 505-511.
16 Rao, A., et al., Palmitoylethanolamide for sleep disturbance. A double-blind, randomised, placebo-controlled interventional study. Sleep Sci Pract, 2021. 5(1): p. 12.
17 Briskey, D., A.R. Mallard, and A. Rao, Increased Absorption of Palmitoylethanolamide Using a Novel Dispersion Technology System (LipiSperse®). Nutraceuticals Food Sci 2020. 5(2:3).



