Georgia Marrion ● 5 min read
The pleiotropic functionality of commensal bacteria, yeasts, protozoa, fungi and archaea present in the gastrointestinal tract is an evolving area of nutritional research.[1-3] Along with well-established roles including promoting intestinal barrier integrity; biosynthesis of energy, vitamins, neurotransmitters and short-chain fatty acids; metabolism of bile acids, xenobiotics and dietary compounds; and regulating metabolism, neurological signaling and endocrine hormone release, the gut microbiota modulate local and peripheral immune system maturation, homeostasis and responsivity.[1-3] As part of ongoing research exploring the players and mechanisms underlying this symbiotic host-bacteria relationship, a group of unique commensal organisms called segmented filamentous bacteria (SFB) have been identified as one such player that significantly influences host adaptive and innate immunity, the gastrointestinal immune barrier and potentially, inflammatory and autoimmune diseases.[3,4]
What are segmented filamentous bacteria?
SFB was initially discovered and characterised in the 1960’s with the provisional name Candidatus arthromitus, following animal research indicating a role of the organism in priming of Th17 cells and a preponderance for autoimmune disease if unabated. SFB are commensal gram-positive, spore-forming organisms genetically similar to the Clostridium genus that adhere to the ileal epithelium of vertebrate hosts. [3-5] Their colonization appears to be age-dependent and predominant below 3 years of age, with the concentration of SFB at 0-6 months, 7-12 months and from 3-75 years of age observed to be 25%, 75% and 6.2% respectively. [4,6,7] The unique characteristics of SFB compared those exhibited by general commensal bacteria are relevant to the subsequent host immune response, specifically the organisms’ morphology, binding mechanism, location and lifecycle, as well as mode of communication with host cells. [3,4]
General commensal bacteria located in intestinal mucosa use indirect modes of communication due to the local presence of mucous, immunoglobulin A and antimicrobial peptides, with mechanisms including secretion of lipopolysaccharides and short chain fatty acids, and modification of host metabolites. [3-5] This contrasts with the more direct bacteria-host communication mechanisms used by SFB known as microbial adhesion-triggered endocytosis (MATE), where cell wall-associated bacterial proteins are commuted into host intestinal epithelial cells, allowing the sampling of commensal antigens, stimulating a subsequent immune response. [5]
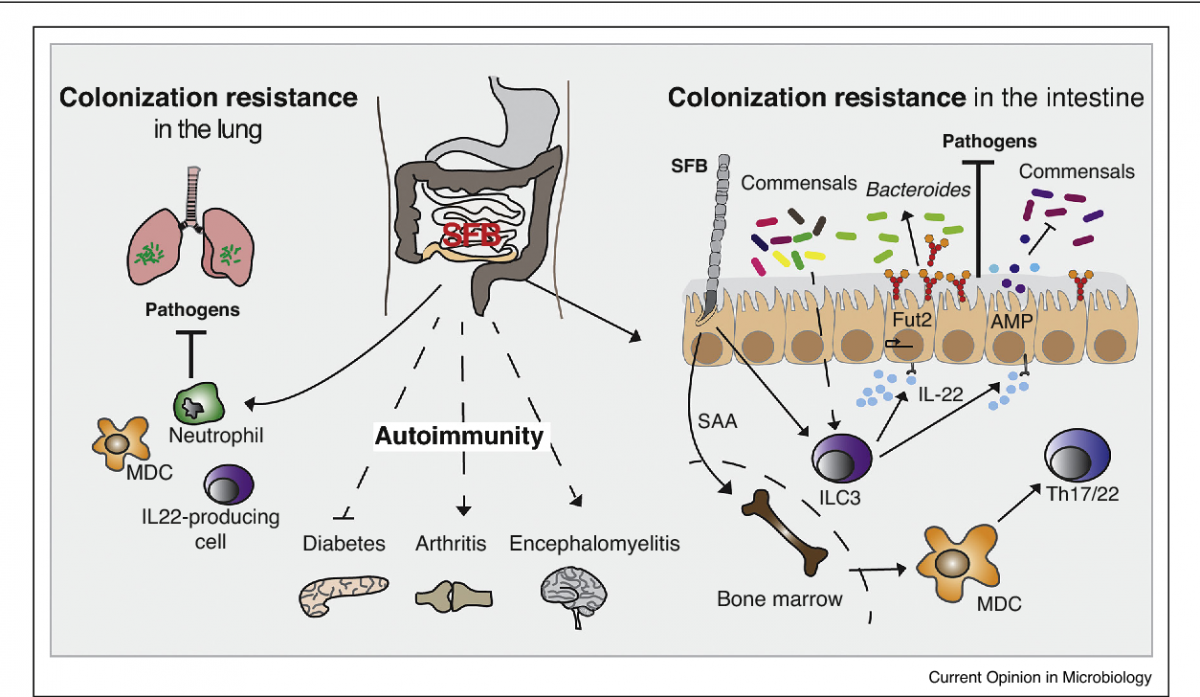
Specifically, SFB promotes activation of innate and adaptive immunity via a number of mechanisms, primarily preferential induction of antigen-specific CD4+ T-helper 17 cells via the transfer of SFB-derived antigens to mesenteric lymph nodes, where antigen-specific naïve T-cells convert to RORgt-expressing cells and subsequently RORgt/IL-17A-expressing cells in the ileum. These Th17 cells stimulate the synthesis of various inflammatory and immune modulating compounds including serum amyloid A, interleukin (IL)-17A, IL-17F, IL-21 and IL-22, and antimicrobial peptides. Other mechanisms include stimulation of faecal secretory IgA levels and activity, and lamina propria concentrations of T-cell receptor intraepithelial lymphocytes, interferon-g and Foxp3-containing CD4+ cells. [2-5,8]
What is the clinical relevance of SFB?
The clinical relevance pertains to the roles of these SFB-induced compounds in inflammatory processes and maintaining mucosal defense integrity against pathogens and toxins via mechanism including modulating neutrophil chemotaxis, activation and differentiation, stimulating antimicrobial peptides, factors and chemokines, ADP-ribosyltransferases synthesis and binding to antigens. [2,4,5,9]
Consequently, SFB is crucial for the development and maintenance of local intestinal mucosal homeostasis and epithelium immune barrier integrity and function against exogenous pathogens, bacterial and fungal pathogens, as well as systemic immune activation. [3,5,10]
These local and systemic effects of SFB underlie both positive and negative associations exhibited by these organisms in ulcerative colitis, psoriasis and autoimmune conditions including rheumatoid arthritis and type 1 diabetes. [6, 11-13]
Investigations to date have demonstrated predominantly positive associations between SFB and animal and human hosts, however, the need for further investigations into SFB are highlighted by animal data demonstrating pathogenic responses induced by SFB. The purported inhibitory mechanism which prevents autoimmune priming is thought to be related to commensal gut bacteria keeping inflammatory priming in check, however further investigations are required to confirm the specific commensals involved. In an animal colitis model, SFB induced a pathogenic Th17/Th1 response in the colon and rectum in the presence of Helicobacter hepaticus, however other investigations have demonstrated that SFB did not promote colitis in the ileum in the absence of inflammation or active disease. [5,13,14] In animal arthritis models, SFB colonisation has been shown to potentiate autoimmune arthritis, induce inflammation, joint thickening and arthritis, and provoke autoantibodies in the lung in the pre-arthritis phase. [15,16] Animal type 1 diabetes models have variably observed that SFB had suppressive or no effect on disease incidence. [13,17] Various researchers have suggested that a key factor potentially influencing if SFB promotes pathogenic or protective effects is the composition of other commensal bacteria. [18]
Much is still to be learnt about SFB, including their presence and prevalence in humans, other potential endogenous mechanisms of action, their role in disease onset or protection and the most appropriate use of SFB for effective clinical outcomes. [2-6]
References:
- Martin AM, Sun EW, Rogers GB, Keating DJ. The influence of the gut microbiome on host metabolism through the regulation of gut hormone release. Front Physiol 2019 Apr; 10: 428.[Abstract]
- Yi J, Jung J, Han D, Surh CD, Lee YJ. Segmented filamentous bacteria induce divergent populations of antigen-specific CD4 T cells in the small intestine. Mol Cells 2019 Mar 31; 42 (3): 228-236.[Absract]
- Schnupf P, Gaboriau-Routhiau V, Sansonetti PJ, Cerf-Bensussan N. Segmented filamentous bacteria, Th17 inducers and helpers in a hostile world. Curr Opin Microbiol 2017; 35: 100-109.[Abstract]
- Hedblom GA, Reiland HA, Sylte MJ, Johnson TJ, Baumler DJ. Segmented filamentous bacteria – metabolism meets immunity. Front Microbiol 2018 Aug 24; 9: 1991. [Full Text]
- Ladinsky MS, Araujo LP, Zhang X, Veltri J, Galan-Diez M, Soualhi S et al. Endocytosis of commensal antigens by intestional epithelial cells regulates mucosa T cell homeostasis. Science 2019 Mar 8; 363 (6431).[Abstract]
- Finotti A, Gasparello J, Lampronti I, Cosenza LC, Maconi G, Matarese V et al. PCR detection of segmented filamentous bacteria in the terminal ileum of patients with ulcerative colitis. BMF Open Gastroenterol 2017 Dec 4; 4 (1): e000172. [Abstract]
- Liu WR, Shu XL, Gu WZ, Peng KR, Zhao H, Chen B, Jiang LQ, Jiang MZ. Age distribution characteristics of intestinal segmented filamentous bacteria and their relationship with intestinal immunity in children. Zhongguo Dang Dai Er Ke Za Zhi 2019 Jun; 21 (6): 534-540. [Abstract]
- Sano T, Huang W, Hall JA, Yang Y Chen A, Gavzy SJ, Lee JY, Ziel JW et al. An Il-23R/IL-22 circuit regulates epithelial serum amyloid A to promote local effector Th17 responses. Cell 2015 Oct; 8: 163 (2): 381-93. [Abstract]
- Brandtzaeg P. Secretory IgA: designed for antimicrobial defense. Front Immunol 2013; doi [Source]
- Chen B, Chen H, Shu X, Yin Y, Li J, Qin J, Chen L et al. Presence of segmented filamentous bacteria in human children and its potential role in the modulation of human gut immunity. Front Microbiol 2018 Jun 29; 9: 1403.[Full Text]
- Flannigan KL, Denning TL. Segmented filamentous bacteria-induced immune responses: a balancing act between host protection and autoimmunity. Immunology 2018 May17.[Abstract]
- Stehilkova Z, Kostovcikova K, Kverka M, Rossmann P, Dvorak J, Novosadova I et al. Crucial role of microbiota in experimental psoriasis revealed by gnotobiotic mouse model. Front Microbiol 2019 Feb 21; 10: 236.[Abstract]
- De Riva A, Walberg M, Ronchi F, Coulson R, Sage A, Thorne L, Goodfellow I, McCoy KD, Azuma M, Cooke A, Busch R. Regulation of type 1 diabetes development and B-cell activation in non-obese diabetic mice by early life exposure to a diabetogenic environment. PLoS One 2017 Aug 3; 12 (8): e0181964.[Abstract]
- Ge Z, Feng Y, Woods SE, Fox JG. Spatial and temporal colonisation dynamics of segmented filamentous bacteria is influenced by gender, age and experimental infection with Helicobacter hepaticus in Swiss Webster mice. Microbes Infect 2015 Jan; 17 (1): 16-22.[Abstract]
- Bradley CP, Teng F, Felix KM, Sano T, Naskar D, Block KE, Huang H, Knox KS, Littman DR, Wu HJ. Segmented filamentous bacteria provoke lung autoimmunity by inducing gut-lung axis Th17 cells expressing dual TCRs. Cell Host Microb 2017 Nov 8; 22 (5): 697-704.[Abstract]
- Sano T, Huang W, Hall JA, Yang Y Chen A, Gavzy SJ, Lee JY, Ziel JW et al. An Il-23R/IL-22 circuit regulates epithelial serum amyloid A to promote local effector Th17 responses. Cell 2015 Oct; 8: 163 (2): 381-93.[Abstract]
- Kriegel MA, Sefik E, Hill JA, Wu HJ, Benoist C, Mathis D. Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proc Natl Acad Sci USA 2011 Jul 12; 108 (28): 11548-11553.[Abstract]
- Stehilkova Z, Kostovcikova K, Kverka M, Rossmann P, Dvorak J, Novosadova I et al. Crucial role of microbiota in experimental psoriasis revealed by gnotobiotic mouse model. Front Microbiol 2019 Feb 21; 10: 236.[Abstract]
DISCLAIMER:
The information provided on FX Medicine is for educational and informational purposes only. The information provided on this site is not, nor is it intended to be, a substitute for professional advice or care. Please seek the advice of a qualified health care professional in the event something you have read here raises questions or concerns regarding your health.