Chronic pelvic pain (CPP) is characterised as pain perceived in the pelvic area, occurring for at least six months duration, irrespective of both menstruation and intercourse. CPP may affect both genders, however, it primarily occurs in women. Globally, up to 26% of women experience CPP for greater than a one-year duration.1
CPP is usually non-gynaecological2 with no pelvic disease identified in approximately ONE THIRD of individuals.3
Pain may originate from any of the urogynecological, gastrointestinal, pelvic, musculoskeletal, neurological, and/ or psychological systems - highlighting that a single-organ pathological approach should be avoided. As with most pain conditions, CPP negatively impacts quality of life and is associated with an increased risk of depression and anxiety, as well as a decrease in sexual satisfaction.4
CPP comorbidities
CPP is commonly associated with several conditions including Irritable bowel syndrome (IBS), endometriosis, and bladder pain syndrome. Often these conditions coexist because of the neuroanatomy of the pelvis. Sensory information from organs within the pelvis such as the rectum, bladder, and vagina converge on the same spinal cord neural circuits resulting in neural cross-sensitisation (see Figure 1).5 For example, there is a two-fold increased risk of IBS in women with endometriosis compared to women without.6
- 1 in 5 in individuals with CPP has IBS
- 1 in 5 individuals with CPP has interstitial cystitis
- 1 in 5 individuals with CPP has depression
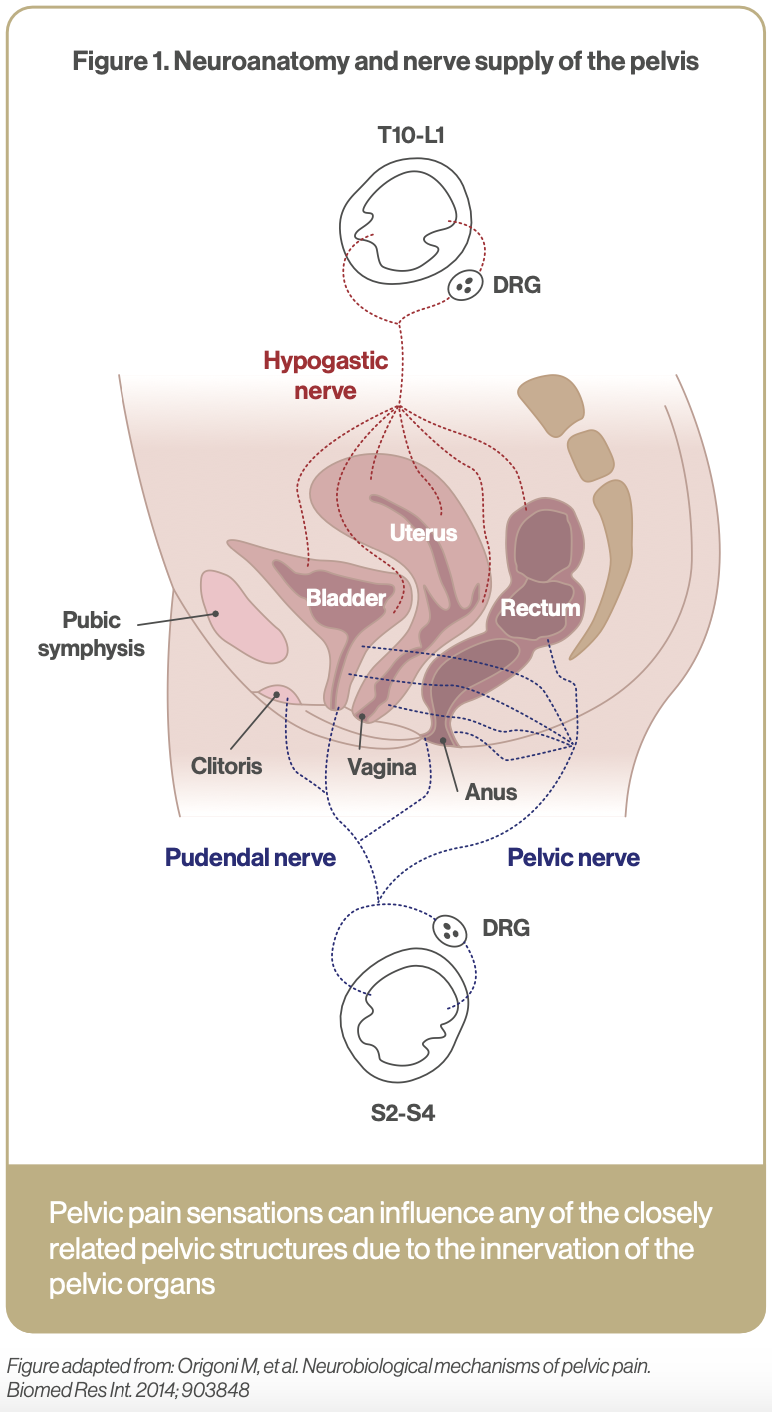
Dysfunctional processing of pain and sensory information can contribute to the development of CPP resulting in a continuous and amplified pain signal being transmitted to the pelvis, often despite injury or inflammation.7
Central Sensitisation: Faulty pain signalling system in CPP
This may occur due to prolonged stimulation of nociceptors leading to mild stimuli being interpreted as painful (hyperalgesia). Holistic management of CPP encompasses a biopsychosocial model that understands that in many cases CPP lacks a somatic driver and may instead be generated by the brain and spinal cord (see Table 1).
Etiopathogenesis
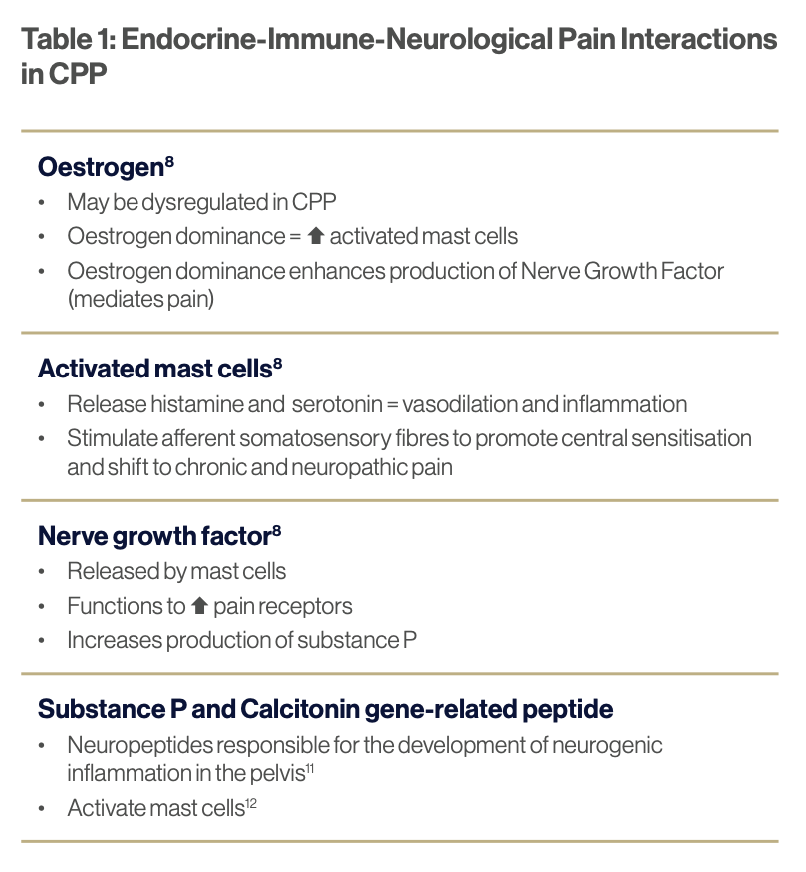
Biological mechanisms underpinning CPP are multifaceted and include inflammatory, nociceptive, neuropathic, and psychogenic factors. Underlying pathophysiology involves a complex interplay between hormones, activated mast cells, inflammatory cytokines, neurotransmitters, endocannabinoids, as well as pro-inflammatory and nociceptive mediators such as substance P and nerve growth factor (NGF).8
Endocrine-Neurological-Immune interactions
Prolonged stress has been shown to worsen CPP, and women with CPP may show alterations in normal HPA axis function.9 Disturbances in the neuroendocrine system may lead to activation of inflammatory cytokines and upregulation
of corticotrophin releasing hormone expressed on mast cells with subsequent release of neuropeptides that promote central sensitisation. Approximately 50% of women with CPP report a history of sexual, physical, or emotional trauma and one-third are positive for Post Traumatic Stress Disorder (PTSD). Practitioners should be aware of applying a trauma-centred approach to care.10
SUPPLEMENTATION
Palmitoylethanolamide (PEA)
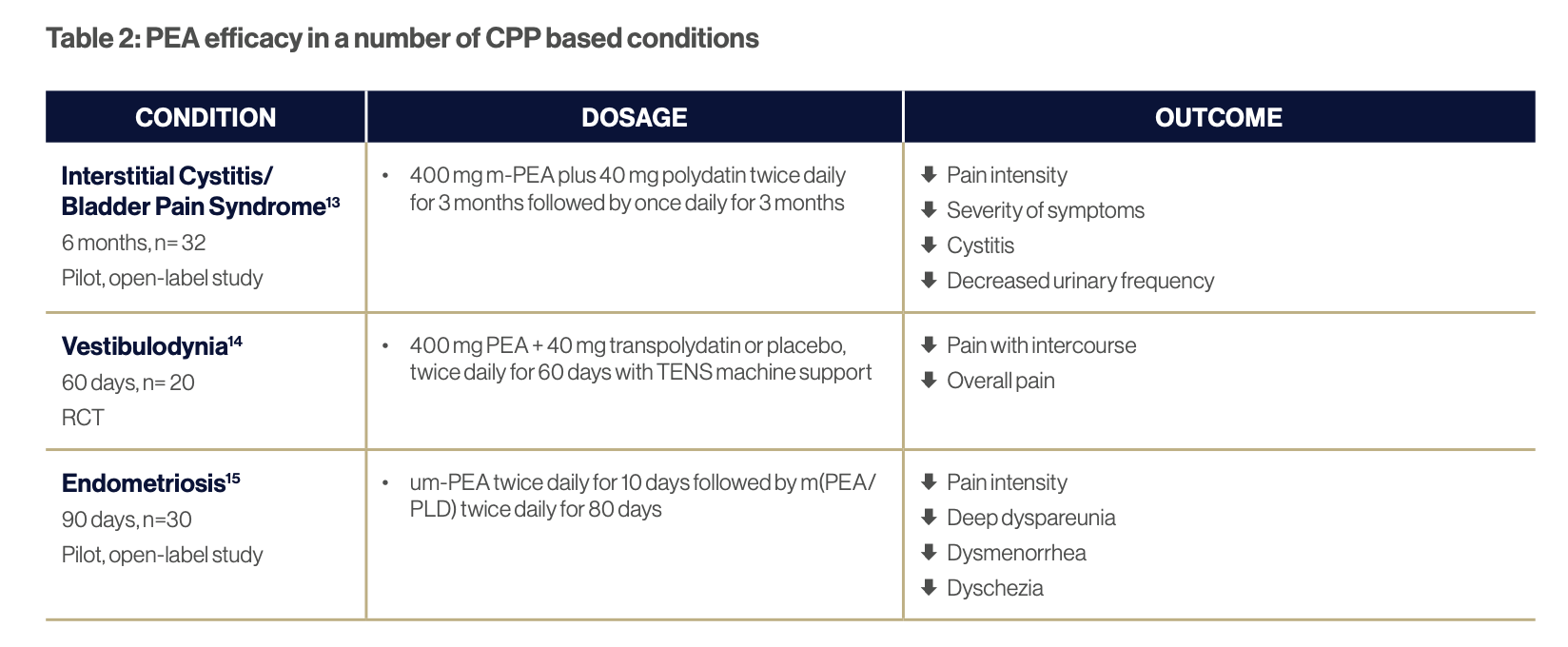
PEA appears to work on the mast cell-glial axis and has shown efficacy in several CPP based conditions (see Table 2). PEA may work for CPP via multiple mechanisms including reducing inflammation, down-regulating mast cell hyperactivity, and decreasing NGF release from nerve cells, thus reducing associated hyperalgesia and allodynia (see Table 2).
Quercetin
Quercetin has been shown to reduce CPP in men, and CPP associated with endometriosis in women. Quercetin demonstrates anti-inflammatory potential however, in vitro studies reveal it may also reduce neuropathic pain via its ability to stabilise mast cells16 making it a useful option for CPP.
Vitamin D
While no studies have been conducted specifically for CPP, vitamin D has been shown to assist with reducing chronic pain in a range of conditions including dysmenorrhea.17,18,19 This may be via multiple mechanisms including reducing inflammation and influencing peripheral and parasympathetic nerve function20 highlighting its role for CPP.
Saffron (Crocus sativus)
Antidepressants are often prescribed for the management of CPP and may function by modifying neural mechanisms of pain. This application provides a rationale for the use of herbal medicines such as saffron which demonstrates antinociceptive, analgesic, and neuroprotective properties. While no specific studies have been undertaken in CPP, saffron has been shown to reduce pain in dysmenorrhea, fibromyalgia, rheumatoid arthritis, and peripheral neuropathy.21 Saffron should be considered as an option for managing CPP as well as comorbid depression.
Anti-Inflammatories - Ginger (Zingiber officinalis), Curcumin (Curcuma longa), and Specialised Pro-Resolving Mediators (SPMs)
Toll-like receptor 4 activation plays a role in increasing inflammatory pain in CPP.22 Anti-inflammatory and immune regulating therapeutics such as Ginger, Curcumin and SPMs may be considered for the management of CPP.
LIFESTYLE
Cognitive Behavioural Therapy (CBT)
Neuroimaging of women with CPP reveals that the brain undergoes changes in morphology in response to constant pelvic pain, specifically loss of grey matter volume in regions involved in pain processing.23 Interestingly, CBT interventions have been shown to increase grey matter volume in areas associated with reducing pain catastrophising and thus may be a useful adjuvant treatment strategy for managing CPP.
Yoga
Reduction in pain severity, improvements in emotional well- being, and sexual function were observed after 6 weeks of Iyengar-based yoga therapy in women with CPP.24 A possible mechanism of action in reducing CPP includes the ability of yoga to increase brain derived neurotrophic factor (BDNF), promoting neuroplasticity and modifying the stress response.25
Tens Machine
Transcutaneous electrical nerve stimulation (TENS) with a pulse duration of 50-400 μs, at a frequency of 2-120 Hz may mildly reduce CPP26 and could be considered as an in-home strategy to manage CCP.

Download a pdf copy of this article
References
1 Arnold MJ, et al. Chronic Pelvic Pain in Women: ACOG Updates Recommendations. Am Fam Physician. 2021 Feb 1;103(3):186-188
2 Lamvu G, et al. Chronic Pelvic Pain in Women: A Review. JAMA. 2021. Jun 15;325(23):2381-2391.
3 Dydyk AM & Gupta N. Chronic Pelvic Pain. [Updated 2023 Apr 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554585/
4 Siqueira-Campos VM, et al. Current Challenges in the Management of Chronic Pelvic Pain in Women: From Bench to Bedside. Int J Womens Health. 2022 Feb 18;14:225-244.
5 Origoni M, et al. Neurobiological mechanisms of pelvic pain. Biomed Res Int. 2014; 903848.
6 Chiaffarino F, et al. Endometriosis and irritable bowel syndrome: a systematic review and meta-analysis. Arch Gynecol Obstet. 2021 Jan;303(1):17-25.
7 As-Sanie S, et al. Incidence and predictors of persistent pelvic pain following hysterectomy in women with chronic pelvic pain. Am J Obstet Gynecol. 2021 Nov;225(5):568
8 Yosef, A. et al. Chronic pelvic pain: Pathogenesis and validated assessment, Middle East Fertility Society Journal, 2016; 21; 205-221
9 Heim C, et al. Abuse-related posttraumatic stress disorder and alterations of the hypothalamic pituitary-adrenal axis in women with chronic pelvic pain. Psychosom Med. 1998 May-Jun;60(3):309-18.
10 Speer LM, et al. Chronic Pelvic Pain in Women. Am Fam Physician. 2016 Mar 1;93(5):380-7
11 Iyengar S, Ossipov MH, Johnson KW. The role of calcitonin gene–related peptide in peripheral and central pain mechanisms
including migraine. PAIN. 2017 Apr;158(4):543–59.
12 Lauritano D, Mastrangelo F, D’Ovidio C, Ronconi G, Caraffa A, Gallenga CE, et al. Activation of Mast Cells by Neuropeptides: The Role of Pro-Inflammatory and Anti-Inflammatory Cytokines. International Journal of Molecular Sciences [Internet]. 2023 Jan 1;24(5):4811. Available from: https://www.mdpi.com/1422-0067/24/5/4811
13 Cervigni M, et al. Palmitoylethanolamide-Polydatin Reduces the Painful Symptomatology in Patients with Interstitial Cystitis/ Bladder Pain Syndrome. Biomed Res Int. 2019 11;9828397
14 Murina F, et al. Vestibulodynia: synergy between palmitoylethanolamide + transpolydatin and transcutaneous electrical nervestimulation. J Low Genit Tract Dis. 2013 Apr;17(2):111-6.
15 Stochino Loi E, et al. Effect of ultramicronized-palmitoylethanolamide and co-micronized palmitoylethanolamide/polydatin on chronic pelvic pain and quality of life in endometriosis patients: An open-label pilot study. Int J Womens Health. 2019;12;11:443-449.
16 Gao W, et al. Quercetin ameliorates paclitaxel-induced neuropathic pain by stabilizing mast cells, and subsequently blocking PKCε- dependent activation of TRPV1. Acta Pharmacol Sin. 2016 Sep;37(9):1166-77
17 Yong WC, et al. Effect of vitamin D supplementation in chronic widespread pain: a systematic review and meta-analysis. Clin Rheumatol. 2017 Dec;36(12):2825-2833
18 Wu Z, et al. Effect of Vitamin D Supplementation on Pain: A Systematic Review and Meta-analysis. Pain Physician. 2016 Sep- Oct;19(7):415-27
19 Zheng SH, et al. Antioxidant vitamins supplementation reduce endometriosis related pelvic pain in humans: a systematic review and meta-analysis. Reprod Biol Endocrinol. 2023 Aug 29;21(1):79
20 Helde-Frankling M, Björkhem-Bergman L. Vitamin D in Pain Management. Int J Mol Sci. 2017 Oct 18;18(10):2170.
21 Pourbagher-Shahri AM, Forouzanfar F. Saffron (Crocus sativus) and its constituents for pain management: A review of current evidence. Phytother Res. 2023 Nov;37(11):5041-5057.
22 Schrepf A, Bradley CS, O’Donnell M, Luo Y, Harte SE, Kreder K, et al. Toll-like Receptor 4 and comorbid pain in Interstitial Cystitis/ Bladder Pain Syndrome: A Multidisciplinary Approach to the Study of Chronic Pelvic Pain research network study. Brain, Behavior, and Immunity. 2015 Oct;49:66–74.
23 As-Sanie S, et al. Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain. 2012;153(5):1006–1014.
24 Huang AJ, Rowen TS, Abercrombie P, Subak LL, Schembri M, Plaut T, Chao MT. Development and Feasibility of a Group-Based Therapeutic Yoga Program for Women with Chronic Pelvic Pain. Pain Med. 2017 Oct 1;18(10):1864-1872
25 Saxena R, Gupta M, Shankar N, Jain S, Saxena A. Effects of yogic intervention on pain scores and quality of life in females with chronic pelvic pain. International Journal of Yoga. 2017 Jan;10(1):9.
26 Babazadeh-Zavieh SS, et al. Effects of Transcutaneous Electrical Nerve Stimulation on Chronic Pelvic Pain in Women: A Systematic Review and Meta-Analysis. Complement Med Res. 2023;30(2):161-173



