Background
Homocysteine is an intermediate produced in the metabolism of sulfur-containing amino acids.[1] Elevated levels of homocysteine are associated with heart attack, heart disease, vascular disease as well as dementia and cognitive decline, macular degeneration, migraine, neural tube defects and bone fractures.[2-5]
In 1968, Harvard researchers observed that children with a genetic defect that caused them to have sharply elevated homocysteine levels suffered severe atherosclerotic occlusion and vascular disorders similar to what is seen in middle-aged patients with arterial disease. This was the !rst indication that excess homocysteine might be an independent risk factor for heart disease.[3]
The thesis that homocysteine was an important determinant in the development of vascular disease attracted little traction until Dr Meir Stampfer and colleagues from Harvard University provided strong evidence using data from the Physicians’ Health Study. They showed that the risk of myocardial infarction was threefold higher in subjects with homocysteine levels in the top 5% of values compared with homocysteine in the bottom 90%.[4]
Although oxidised LDL cholesterol is commonly considered the arteries’ worst enemy, homocysteine is considered an equally, if not more, powerful threat to heart health. Research now shows that damage from homocysteine paves the way for LDLs to have a more destructive effect on the vascular system.
How is homocysteine measured?
Homocysteine is measured through a routine blood test.
Occasionally, the methionine-load test may also be done. This test measures homocysteine levels before and after the intake of 100mg/kg of methionine (dissolved in orange juice). The test is most commonly used to diagnose abnormal homocysteine metabolism in people who have a high risk for cardiovascular disease, but who have normal baseline homocysteine levels. This test can be used to make decisions about therapy, as people with abnormal “load” tests may respond better to vitamin B6 supplementation compared with folic acid.[2]
What are considered elevated homocysteine levels?
“Normal” and “abnormal” values may be set by individual laboratories. Typically, a level less than 13mcmol/L is considered normal. A level between 13-60mcmol/L is considered moderately elevated, and a value greater than 60-100mcmol/L is severely elevated.[2]
Causes of high homocysteine
Below are some of the influences and implications of elevated levels of homocysteine.[2-6]

Treatments of high homocysteine
- B vitamins – B2, B6, folic acid, B12
- Serine
- Taurine
- Choline
- S-adenosylmethionine (SAMe)
- N-acetyl-cysteine (NAC)
- Omega-3
Mechanisms of action
Homocysteine is metabolised through two distinct pathways, remethylation and transsulfuration. Each pathway in the below diagram is dependent upon a number of nutrients and enzymes.
Folate, B12 and B2 in the remethylation pathway
The remethylation pathway actually involves methylation, re-methylation and de-methylation reactions involving folate, B12 and B2.
Folic acid coming in from the diet is reduced to dihydrofolate (DHF), which is then reduced again by dihydrofolate reductase (DHFR) to form tetrahydrofolate (THF).
THF is coming from two places: from the reduction of dietary folate; and from active folate or 5-methyl-tetrahydrofolate (5-MTHF) donating its methyl group to homocysteine. THF is methylated by serine, requiring pyridoxal-5-phosphate (P-5-P or B6). THF becomes 5,10-methylene-tetrahydrofolate and glycine is produced from the now de-methylated serine.
The 5,10-methylene-THF now needs to be reduced by 5,10-methylene-tetrahydrofolate reductase (MTHFR) enzyme to produce active folate (5-MTHF). This 5-MTHF then donates its methyl group to homocysteine, catalysed by methionine synthase (a vitamin B12 dependent enzyme).
This methylation of homocysteine produces methionine and results in 5-MTHF becoming THF again, which then needs to be re-methylated again by serine, and the cycle continues.
Methionine is converted to SAMe, which acts as a major methyl donor in a range of other reactions, yielding the de-methylated s-adenosylhomocysteine (SAH) which is converted to homocysteine (by hydrolysis) and requires re-methylation by 5-MTHF to become methionine again.
By supplementing with folinic acid (5-formyl-THF), a number of reduction steps can be bypassed to convert directly to 5,10-methylene-THF, alleviating the need for DHFR enzyme.
Riboflavin (B2) is an important nutrient supporting the MTHFR enzyme, which is involved in the activation of folate into 5-methyl-THF. Studies have shown that plasma riboflavin is a determinant of plasma homocysteine levels.[7,8]
B6 and serine in the transsulfuration pathway
Homocysteine can also go down the transsulfuration pathway where it is converted into cystathione, requiring P-5-P (B6) and serine, then into cysteine and ultimately glutathione, an important and powerful antioxidant.
Lowering homocysteine levels
Some researchers believe homocysteine is an indicator of folate and/or B12 deficiency. There have been a number of studies looking at the effects of supplementation with B group vitamins in the treatment of elevated levels of homocysteine.
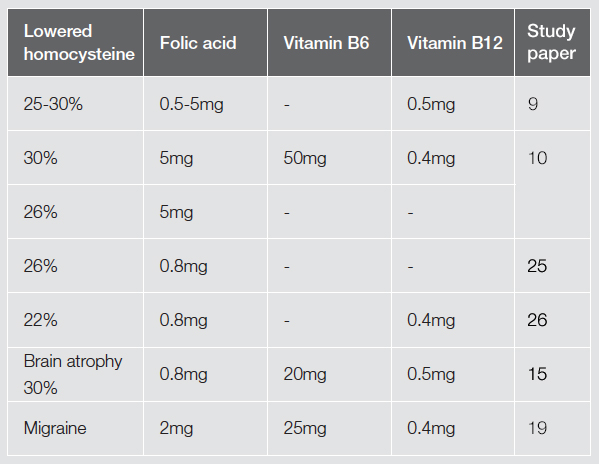
A meta-analysis of 12 randomised trials looking at supplements to lower homocysteine levels found that folic acid supplementation of 0.5-5mg lowered homocysteine levels by 25%; vitamin B12, with an average dose of 0.5mg, produced an additional reduction of 7%; whereas B6 (average 16.5mg) did not have a significant effect. The authors concluded that in typical populations daily supplementation with folic acid and B12 would be expected to reduce homocysteine levels by a quarter to a third.[9]
One study looked at the effect of vitamin supplementation on plasma homocysteine, finding that the most effective results came from a combination of nutrients: 5mg of folic acid, 0.4mg of hydrocobalamin and 50mg of pyridoxal. This combination reduced homocysteine levels by an average of 30%. Folic acid alone was found to achieve a mean reduction of homocysteine of 26%, and combined vitamin supplementation reduced homocysteine levels effectively in patients with venous thrombosis and in healthy volunteers, either with or without hyperhomocysteinemia.[10]
Cardiovascular disease
Elevated homocysteine levels is associated with an increased risk for developing atherosclerosis, which can in turn lead to coronary artery disease (CAD), heart attack and stroke.[2] Epidemiological studies have unequivocally established that elevated plasma homocysteine levels both predicts and precedes the occurrence of cardiovascular disease.[11]
The consequences of hyperhomocysteinemia arise from endothelial cell injury by increased oxidative stress, reduced bioavailability of nitric oxide, increased platelet adhesiveness and enhanced LDL deposition.[12]
Homocysteine binds to certain proteins in the body, affecting their structure and function and inhibiting the repair and maintenance of cartilage, elastin and proteolgycans; making them more susceptible to disease processes.
Homocysteine can damage artery walls; it causes a reduction in nitric oxide activity, impairing the ability of blood vessels to dilate and leaving them susceptible to oxidative damage. Damaged vascular walls allow more LDLs to be absorbed, increases platelet adhesiveness and activates the clotting cascade, therefore narrowing the blood vessels and increasing risk of heart attack or stroke.[5]
The Nurses’ Health Study, the longest running investigation of factors influencing women’s health, demonstrated that B6 and folic acid conferred greater protection as a duo than when used alone. The risk of heart attack or death from heart disease was nearly halved among women who consumed the most folic acid and B6 from diet and supplements compared with those who consumed the least. Smaller but still significant effects occurred with folic acid or vitamin B6 alone.[13]
Elevated homocysteine confers a graded risk with no threshold, is independent of, but may enhance, the effect of the conventional risk factors, and seems a particularly strong predictor of cardiovascular mortality.[11]
Dementia and cognitive decline
It has been recognised that elevated homocysteine is associated with dementia, particularly Alzheimer’s disease. It is suspected that there is a connection between homocysteine levels and blood vessel changes in the brain.
A study concluded that high homocysteine is considered a strong independent risk factor for dementia and Alzheimer’s disease, with a plasma level of greater than 14µmol/L nearly doubling the risk of Alzheimer’s disease.[14]
A recent research project, Oxford Project to Investigate Memory and Ageing (OPTIMA), at Oxford University looked at the effects of B vitamins in the treatment of cognitive decline in the elderly. It was found that an accelerated rate of brain atrophy in elderly patients with mild cognitive impairment can be slowed by treatment with homocysteine-lowering B vitamins. The nutrients used were 0.8mg folic acid, 20mg B6 and 0.5mg B12 a day. The rate of atrophy per year in the treatment group was 0.78% compared with 1.08% in the placebo group. The results of this research also demonstrated that a greater rate of atrophy was associated with lower final cognitive scores.[15]
Women’s health
Elevated homocysteine levels have been observed more frequently among women with pregnancy complications such as preeclampsia, placental abruption, recurrent pregnancy loss, and giving birth to a small, low-birth-weight baby (intrauterine growth restriction).[16]
Hyperhomocysteinemia is seen more commonly in women who give birth to a baby with a neural tube defect. Neural tube defects include spina bifida and anencephaly. Approximately 20% of women who have a child with a neural tube defect have abnormal homocysteine metabolism.[17]
Migraine
Elevated blood levels of homocysteine have been associated with migraine.[18,19] A recent study demonstrated that homocysteine lowering treatment which reduced high levels of homocysteine (25mg B6, 2mg folic acid and 400mcg B12), also decreased the frequency and/or severity of migraines in sufferers by half. The results of this study demonstrated that supplementation with folic acid, B6 and B12 reduced migraine disability, frequency and pain severity.[20]
Macular degeneration
Studies have associated high levels of homocysteine with an increased risk of age-related macular degeneration (AMD). In one study, 2335 participants who had evidence of AMD were reviewed for their homocysteine levels. The results found that participants who were less than 75 years old and had homocysteine levels greater than 15µmol/L had a higher risk of developing AMD.[21]
Further work confirms the relationship with high homocysteine levels and vitamin B12 and folate deficiency and greater risk of early and any age-related macular degeneration.[22]
Bone fractures
Elevated homocysteine levels and low vitamin B12 and folate levels are associated with deteriorated bone health. There are several suggested mechanisms for the association between vitamin B12, folate, homocysteine and bone health.
Homocysteine may interfere with collagen cross-linking. Cross-links are important for the strength and stability of the collagen network. Interference in cross-link formation would cause an altered bone matrix resulting in more fragile bones.23 Collagen cross-links do not affect bone mineral density, which means that traditional measures to build bone health (weight bearing exercise, adequate calcium and vitamin D) will not address the damage that homocysteine causes to bone health.[5,23]
Genetic predisposition
Some people have a genetic variation in the MTHFR gene that can make them more predisposed to developing high homocysteine levels.
The MTHFR gene is responsible for producing an enzyme that helps to regulate homocysteine in the body. Although these mutations do impair the regulation of homocysteine, adequate folate levels can cancel out this defect.[2]
Regardless of whether patients have an MTHFR mutation or not, the treatment for elevated homocysteine is the same—dietary intervention and supplementation with folic acid, B6 and B12. The amount of each of these supplements should be adjusted on the basis of the degree of homocysteine elevation, not genetic status. If patients have mutations in both MTHFR genes but have normal homocysteine levels, they do not need to be on folic acid, B6 or B12 therapy.[2,24]
Clinical studies
Homocysteine’s effect on family history of premature ischaemic stroke
Background: Family history of stroke is an independent risk factor for cardiovascular disease (CVD).
Subjects/Method: The study involved 344 healthy individuals, including 143 with family history of premature ischaemic stroke (PIS) and 201 without (control group).
Intervention: An evaluation of selected metabolic risk factors and an association between the interaction of family history of PIS and homocysteine levels with other risk factors in individuals with family history of PIS.
Results: In the group with family history of PIS, a significantly higher mean body mass index (BMI), systolic and diastolic blood pressure, total cholesterol, LDL cholesterol, apolipoprotein B, apolipoprotein A-I and glucose values were observed. There was a significant interaction of family history of PIS with homocysteine for total cholesterol, HDL cholesterol and triglycerides, with higher homocysteine levels associated with significantly higher values of total cholesterol and triglycerides. Men and women with family history of PIS are characterised by an unfavourable shift in the risk factor profile. This effect is additionally enhanced by higher homocysteine levels, which might be an indication for primary prevention in these individuals.
Risk of bone fracture with high homocysteine
Background: Osteoporosis is a major health problem that is characterised by low bone mineral density, deterioration of bone microarchitecture and an increased risk of fracture. It has been hypothesised that the metabolism of homocysteine is involved in osteoporosis.
Subjects/Method: Looked at the association between circulating homocysteine levels and the risk of osteoporotic fractures in 2406 men and women aged over 55 years over a two-eight year period.
Intervention: A fracture in any skeletal location was documented as an outcome measure by physicians.
Results: The results showed that homocysteine was a strong independent risk factor for osteoporotic fractures in both older men and women.
Folic acid’s effect on cognitive function
Background: Low folate and raised homocysteine concentrations in blood are associated with poor cognitive performance in the general population. As part of the FACIT trial, to assess the effect of folic acid on markers of atherosclerosis in men and women aged 50-70 years with raised plasma total homocysteine and normal serum vitamin B12 at screening, this reports the findings for the secondary endpoint: the effect of folic acid supplementation on cognitive performance.
Subjects/Method: Randomised, double-blind, placebo-controlled study of 818 participants.
Intervention: Received 800mcg folic acid daily or placebo for three years.
Results: Serum folate increased 576% and plasma total homocysteine decreased 26% in folic acid group. The three year change in cognitive function (memory, information processing speed and sensorimotor speed) that tend to decline with age were significantly better in the folic acid group than placebo.
Dosage range for treatments according to clinical studies
Additional considerations
- Decrease methionine-rich foods, particularly red meat and dairy products
- Avoid alcohol, coffee and smoking
- Maintain a healthy weight
- Partake in daily exercise
References
- Higdon J. An evidence-based approach to vitamins and minerals. Thieme, 2003.
- Varga EA, Sturm AC, Misita CP, et al. Homocysteine and MTHFR mutations: relation to thrombosis and coronary artery disease. Circulation 2005;111:e289-e293. [Full text]
- Homocysteine reduction. Life Extension, 2013.Viewed 21 October 2015, http://www.lef.org/protocols/heart_circulatory/homocysteine_reduction_01.htm
- McCarty MF, Thomas CA. The vascular toxicity of homocysteine and how to control it. The Linus Pauling Institute, 1999. Viewed 21 October 2015, http://lpi.oregonstate.edu/f-w99/vascular.html
- Barker JE. Homocysteine. Its destructive role in cardiovascular, cognitive and bone health. Complementary Prescriptions, 2013. Viewed 21 October 2015, http://www.cpmedical.net/articles/homocysteine-its-destructive-role-in-cardiovascular-cognitive-and-bone-health
- Elias AN, Eng S. Homocysteine concentrations in patients with diabetes mellitus - relationship to microvascular and macrovascular disease. Diabetes, Obesity and Metabolism 2005;7:117-121.
- Moat SJ, Ashfield-Watt PA, Powers HJ, et al. Effect of riboflavin status on the homocysteine-lowering effect of folate in relation to the MTHFR (C677T) genotype. Clin Chem 2003;49(2):295-302. [Full text]
- Hustad S, Ueland PM, Vollset SE, et al. Riboflavin as a determinant of plasma total homocysteine: effect modification by the methylenetetrahydrofolate reductase C677T polymorphism. Clin Chem 2000;46(8 Pt 1):1065-1071. [Full text]
- Clarke R, Armitage J. Vitamin supplements and cardiovascular risk: review of the randomized trials of homocysteine-lowering vitamin supplements. Semin Thromb Hemost 2000;26(3):341-348. [Abstract]
- den Heijer M, Brouwer IA, Bos HJ, et al. Vitamin supplementation reduces blood homocysteine levels: a controlled trial in patients with venous thrombosis and healthy volunteers. Arterioscler Thromb Vasc Biol 1998;18(3):356-361. [Full text]
- Refsum H, Ueland PM, Nygard O, et al. Homocysteine and cardiovascular disease. Ann Rev Medicine 1998;49:31-62. [Abstract]
- Dwivedi MK, Tripathi AK, Shukla S, et al. Homocysteine and cardiovascular disease. Biotechnology and Molecular Biology Reviews 2011;6(5):101-107. [PDF]
- B vitamins and homocysteine. Harvard Health Publications. Harvard Medical School 2000-2013. Viewed 25 Oct 2015, http://www.health.harvard.edu/newsweek/B_vitamins_and_homocysteine.htm
- Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N Engl J Med 2002;346(7):476-483. [Full text]
- Smith AD, Smith SM, de Jager CA, et al. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PLoS ONE 2010;5(9):e12244. [Full text]
- Ray JG, Laskin CA. Folic acid and homocyst(e)ine metabolic defects and the risk of placental abruption, pre-eclampsia and spontaneous pregnancy loss: a systematic review. Placenta 1999;20:519-529. [Abstract]
- Botto LD, Yang Q. 5,10-Methylenetetrahydrofolate reductase gene variants and congenital anomalies: a HuGE review. Am J Epidemiol 2000;151:862-877. [Full text]
- Kurth T, Ridker PM, Buring JE. Migraine and biomarkers of cardiovascular disease in women. Cephalalgia 2008;28(1):49-56. [Abstract]
- Moschiano F, D'Amico D, Usai S, et al. Homocysteine plasma levels in patients with migraine with aura. Neurol Sci 2008;29 Suppl 1:S173-S175. [Abstract]
- Lea R, Colson N, Quinlan S, et al. The effects of vitamin supplementation and MTHFR (C677T) genotype and migraine disability. Pharmacogenet Genomics 2009;19(6): 422-428. [Abstract]
- Rochtchina E, Wang JJ, Flood VM, et al. Elevated serum homocysteine, low serum vitamin B12, folate and age related macular degeneration: the Blue Mountains eye study. Am J Ophthalmol 2007; 143(2):344-346. [Abstract]
- Gopinath B, Flood VM, Rochtchina E, et al. 2013.Homocysteine, folate, vitamin B-12, and 10-y incidence of age-related macular degeneration. Am J Clin Nutr 2013;98(1):129-135. [Full text]
- van Wijingaarden JP, Doets EL, Szczecinska A, et al. Vitamin B12, folate, homocysteine, and bone health in adults and elderly people: a systematic review and meta-analysis. J Nutr Metab 2013;2013:486186. [Full text]
- Dell’edera D, Tinelli A, Milazzo GN, et al. Effect of multivitamins on plasma homocysteine in patients with the 5,10 methylenetetrahydrofolate reductase C677T homozygous state. Mol Med Rep 2013;8(2):609-612. [Full text]
- Durga J, et al. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet 2007;369(9557):208-216. [Full text]
- Loland KH, Cleie O, Blix AJ, et al. Effect of homocysteine-lowering B vitamin treatment on angiographic progression of coronary artery disease: a western Norway B vitamin intervention trial (WENBIT) study. Am J Cardiol 2010;105:1577-1584. [Abstract]
DISCLAIMER:
The information provided on FX Medicine is for educational and informational purposes only. The information provided on this site is not, nor is it intended to be, a substitute for professional advice or care. Please seek the advice of a qualified health care professional in the event something you have read here raises questions or concerns regarding your health.



