Even though the liver is considered the primary organ of detoxification, the integrity of the intestinal epithelial barrier must not be underestimated. The gastrointestinal tract (GIT) is a major site for dealing with toxic burden. Over the course of a lifetime, the GIT processes more than 25 tonnes of food, which represents the largest load of antigens and toxins confronting the human body.[1]
Restoring and supporting the integrity and function of the intestinal barrier is an essential, and often first, step in a successful detoxification program.
Under normal circumstances, protective mechanisms within the gut facilitate the absorption of nutrients while blocking the entry of potentially harmful substances. However, a breakdown in the intestinal epithelial lining, also known as “leaky gut”, allows the passage of undesirable molecules into the bloodstream which places the liver under a great deal of stress.
Detoxification itself, is a chain of events. When a toxic molecule enters the body, it is typically sent to the liver for processing. Hence, the liver is often considered the primary organ of detoxification. Once a molecule has been processed in the liver, it is returned into circulation to be removed via the kidneys or through the intestines (via bile) where it is eliminated with the faeces.
If any links in this chain are missing or impaired, the elimination of toxins becomes inefficient and absorbed toxins are retained by the body.
Also involved in detoxification processes of the body are the lungs (breath), skin (sweat) and the lymphatic system.
Liver Detoxification
One of the key functions of the liver is filtration of the blood; this process serves to clear the blood of large molecules, such as bacteria, bacterial endotoxin and antigen-antibody complexes. The liver is also responsible for the synthesis and secretion of bile, which functions as the the vehicle carrying many toxic substances into the intestines for excretion.
The liver enzymatically disassembles undesired chemicals; a process that occurs in two steps known as phase 1 and phase 2 detoxification.
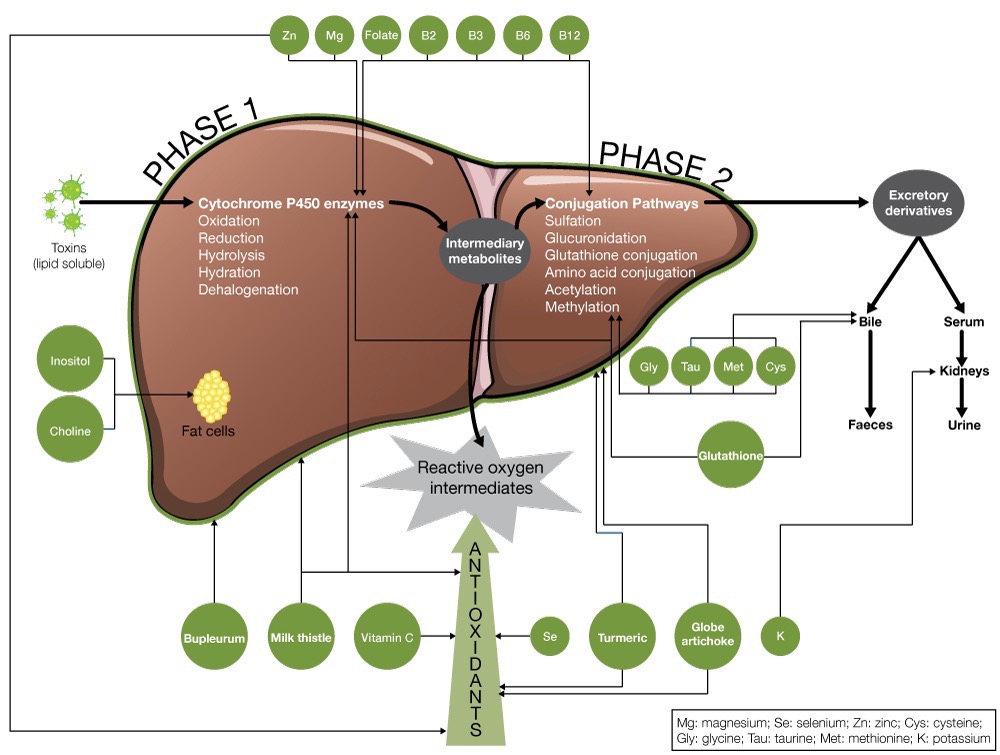
Phase 1 Detoxification
Phase 1 detoxification involves a family of enzymes that are collectively referred to as cytochrome P450 (CYP450). These phase 1 enzymes either directly neutralise a toxin or modify it to form an active intermediate that is then neutralised by phase 2 detoxification proceses.
The activity of phase 1 enzymes can vary significantly from one individual to another, based on their genetic makeup, level of exposure to toxins, some drugs and of course, nutritional status.
Clinically, a noteworthy side-issue with phase 1 detoxification is the consequent generation of free radicals. Without adequate antioxidant defences tissue damage may occur. It is for this reason that antioxidants – especially those that concentrate in the liver – are vitally important.
Phase 2 Detoxification
Most chemicals processed by phase 1 enzymes are converted to intermediary forms that need to be processed by phase 2. These intermediate forms are often more chemically active and therefore more toxic, making a rapid phase 2 response of utmost importance. If the phase 2 detoxification systems are not working adequately, these intermediates can cause substantial damage.
There are six primary phase 2 detoxification pathways: glutathione conjugation, amino acid conjugation, methylation, sulfation, acetylation and glucuronidation. These enzyme systems are particularly dependent on, and responsive to, an adequate supply of substrates – predominantly amino acids – necessary to carry out the conjugation of oxidised metabolites.
Glutathione Conjugation
A primary route of phase 2 detoxification is glutathione conjugation. In addition to its role in conjugation, glutathione (GSH) is a significant hepatic antioxidant. If GSH is depleted by the oxidative by-products of phase 1, the glutathione-dependent phase 2 activity is inhibited. GSH depletion has been suggested to represent an important contributory factor to oxidative liver damage and enhanced morbidity related to liver hypo function.[2]
GSH is a tripeptide made up from the amino acids glutamine, glycine and cysteine, thus an adequate supply of these substrates is paramount.
Foods from the brassica family (cabbage, broccoli, Brussel sprouts) are inducers of this pathway. Broccoli sprouts in particular have been found to be potent phase 2 inducers.[3]
Sulforaphane (active constituent in broccoli and other brassica vegetables), as well as Curcuma longa enhance the activity of glutathione S-transferase (GST).[4,5]
Amino Acid Conjugation
Glycine (and to a lesser extent taurine, glutamine, arginine and ornithine) are required for phase 2 amino acid conjugation.
People consuming a low protein diet, and those with chronic exposure to toxins, are at risk of impaired amino acid conjugation due to substrate depletion, particularly glycine.[6]
Methylation
Methylation is the conjugation of toxins with methyl groups. The methyl donor is S-adenosylmethionine (SAMe). SAMe is synthesised from the amino acid methionine, a process which requires the nutrients choline, vitamin B6, vitamin B12 and folic acid.[6]
Sulfation
Sulfation involves the conjugation of toxins with sulfur-containing compounds. Nutrients needed to maintain sulfation are the sulfur-containing amino acids, particularly cysteine and methionine. An adequate level of molybdenum is also necessary for this phase 2 process.[6]
Acetylation
Acetylation is the conjugation of toxins with acetyl-CoA. Acetylation is dependent on the vitamins B1, B5 and vitamin C.[6]
Glucuronidation
Glucuronidation combines toxins with glucuronic acid; this process depends on adequate levels of glucuronic acid.
Glucuronidation has been shown to be enhanced with green tea administration.[5] Fish oils and limonene from the peel of oranges, lemons, limes and grapefruits may also activate glucuronidation.[6]
Phase 3 detoxification
The third phase of liver detoxification involves the function of specific antiporter proteins. This step is responsible for pumping toxins out of the cells for excretion. This may occur after or prior to detoxification. For example, antiporter proteins are expressed in GIT enterocytes. When certain toxic compounds in food are taken up into the enterocyte, the antiporters “pump” these back into the lumen, preventing their passage before having the opportunity to be “deactivated” by CYP450 enzymes.
This is not a perfect process and some toxins are allowed passage from the lumen into the body. However, supporting the function of these proteins can minimise internal exposure to toxins and reduce the pressure placed on other detoxification organ systems (e.g. liver, kidney, skin).
Elimination of toxins
Vigorous flow of bile
One of the primary routes for the elimination of modified toxins is through the bile. Once the liver has processed toxins, most waste molecules are placed in the bile and sent to the gallbladder en route for fecal excretion. If the flow of bile is inhibited (cholestasis), toxins cannot be efficiently removed.
Lipotropic nutrients, such as choline, methionine, folic acid and the vitamins B6 and B12, are useful to promote the flow of bile from the liver.[6] Several herbs may also stimulate the synthesis and/or flow of bile.
Colonic elimination: the importance of fibre
In the intestine, bile and its toxic load need to be rapidly absorbed by fibre to be excreted via the faeces. Fibre decreases stool transit time, acts to sequester conjugated xenobiotics and endobiotics located in the bile, and reduces the level of bacterial deconjugating enzymes in the stool. All of these effects reduce the recycling of toxins through the enterohepatic circulation.[7]
The gastrointestinal system is both exposed to incoming toxins via ingestion as well as having the pre-metabolised toxins reintroduced into the intestinal environment on their path to excretion. This is why it is so important to not only minimise toxin exposure and improve the availability of nutrients and antioxidants, but also support optimal barrier function and bowel regularity.
Supporting intestinal barrier function
There are numerous modifiable causes of intestinal hyperpermeability including nutritional deficiencies, consumption of junk food, gluten and wheat (in sensitive individuals and/or due to over-consumption), excessive consumption of sugary foods, food allergies, severe emotional stress or trauma, medications (such as antibiotics and anti-inflammatory drugs), alcohol abuse, gastrointestinal parasites, intestinal infections or microbial overgrowth and radiation.
Increased intestinal permeability also occurs with certain disease states such as inflammatory bowel disease, rheumatoid arthritis, ankylosing spondylitis, asthma, eczema and following trauma or surgery.[8]
The role of the gastrointestinal tract in detoxification
The role of the GIT cells must be considered when aiming to reduce an individual’s accumulation of both external and endogenously derived toxins. It is estimated that 25% of detoxification processes occur within GIT enterocytes. These cells carry out both phase 1 (via the action of CYP450 enzymes) and phase 3 (via antiporter protein action) functions in an attempt to reduce the passage of non-metabolised xenobiotics to the internal environment.
A large portion of the toxins we are exposed to on a daily basis enter the body within foods and beverages we consume. If intestinal hyperpermeability (“leaky gut”) is present, this allows greater entry of undesirable compounds into the body, many of which can produce “toxicity” symptoms once they reach a certain threshold. In addition, leaky gut allows these potentially toxic compounds to by-pass the processes of detoxification that would normally be carried out within the gut cells.
Furthermore, as the GIT is a major site of metabolised toxin excretion (originating from the liver), compromised intestinal integrity, combined with insufficient fibre and dysbiosis, may also lead to reabsorption of toxin metabolites otherwise bound for elimination.
Research also shows local inflammation in gut cells can result in glutathione depletions which in turn may compromise antiporter protein (i.e. phase 3) function. Correcting dysbiosis with a broad spectrum, high dose probiotic, together with dietary prebiotic fibres and additional nutrients including L-glutamine, can help not only to reduce local inflammation, but also to restore integrity to the GIT. Moreover, beneficial micro-organisms themselves assist detoxification and protection against toxicity by binding/ sequestering damaging compounds (e.g. heavy metals).
Several nutrients may help maintain mucosal integrity and reverse hyperpermeability.
REFERENCES
- Liska DJ. The detoxification enzyme systems. Altern Med Rev 1998;3(3):187-198. [Full Text]
- Kidd PM. Glutathione: systemic protectant against oxidative and free radical damage. Altern Med Rev 1997;2(3)155-176. [Full Text]
- Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci U S A 1997;94(19):10367-10372. [Full Text]
- Crinnion WJ. Do environmental toxicants contribute to allergy and asthma? Altern Med Rev 2012;17(1):6-18. [Full Text]
- Luper S. A review of plants used in the treatment of liver disease: part 2. Altern Med Rev 1999;4(3):178-189. [Full Text]
- Murray MT, Pizzorno JE. Textbook of natural medicine, 2nd ed. Churchill Livingstone, 1999.
- Lyon MR. Functional toxicology. In: Pizzorno JE, Murray MT (eds.). Textbook of natural medicine, 3rd ed (pp. 593-607). St Louis, Missouri: Churchill Livingstone Elsevier, 2006.
- Miller AL. The pathogenesis, clinical implications and treatment of intestinal hyperpermeability. Altern Med Rev 1997;2(5):330-345. [Full Text]
DISCLAIMER:
The information provided on FX Medicine is for educational and informational purposes only. The information provided on this site is not, nor is it intended to be, a substitute for professional advice or care. Please seek the advice of a qualified health care professional in the event something you have read here raises questions or concerns regarding your health.