The Clinical Use of Phosphatidylcholine
Phosphatidylcholine (PC) is a phospholipid integral to maintaining the structure and fluidity of a cell membrane. Additionally, PC provides structure to circulating lipoproteins and is essential for lipid transport and metabolism.1 PC is as an essential component of bile, facilitating fat emulsification, absorption and transport and is an important constituent of surfactants in the body, including those of the lungs and gastrointestinal tract, offering protection to epithelial-luminal interfaces.2
The abundance of PC within cellular membrane bilayers and organelles, and the intercellular variability in PC concentrations between different cell types, is representative of its physiological importance in the body.3,4 Comprising 40-50% of total cellular phospholipids, the clinical significance of PC’s physiological presence is highlighted by ongoing research into links between endogenous PC levels and its mechanistic functions, with pathologies involving several body organs and systems.4
What is Phosphatidylcholine?
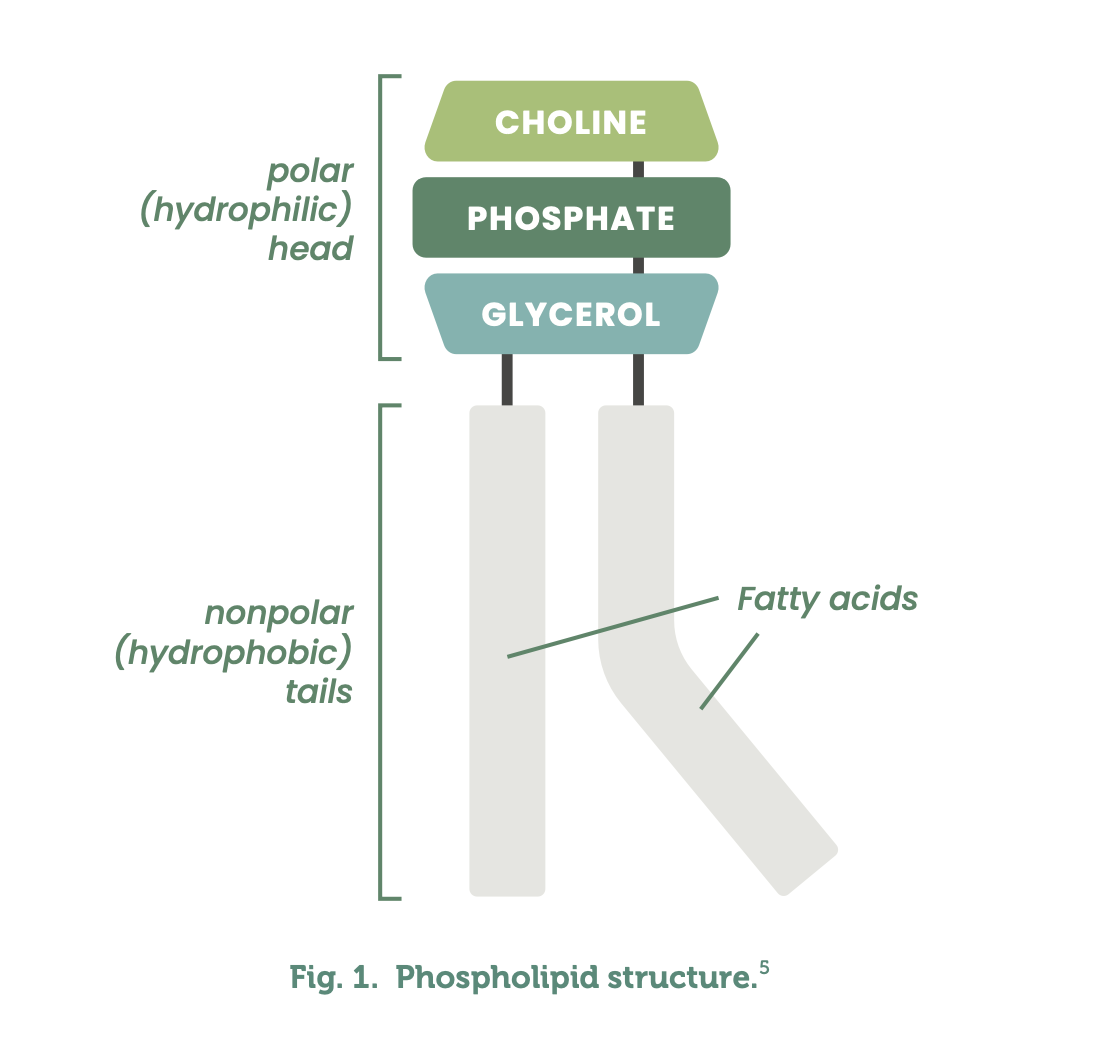
Composed of two hydrophobic fatty acyl chains and a glycerol hydrophobic head, phospholipids are found in cellular membranes, offering structural support to the cell.3 Phosphatidylcholine is a phospholipid with a phosphate group joined to a molecule of choline at the hydrophobic head, differentiating it from other phospholipids.4 (See Fig. 1)
Sometimes referred to as lecithin although technically different, PC is in fact a component of lecithin.5 Acetylcholine, essential for memory, is produced by the body from phosphatidylcholine.5
Phosphatidylcholine synthesis
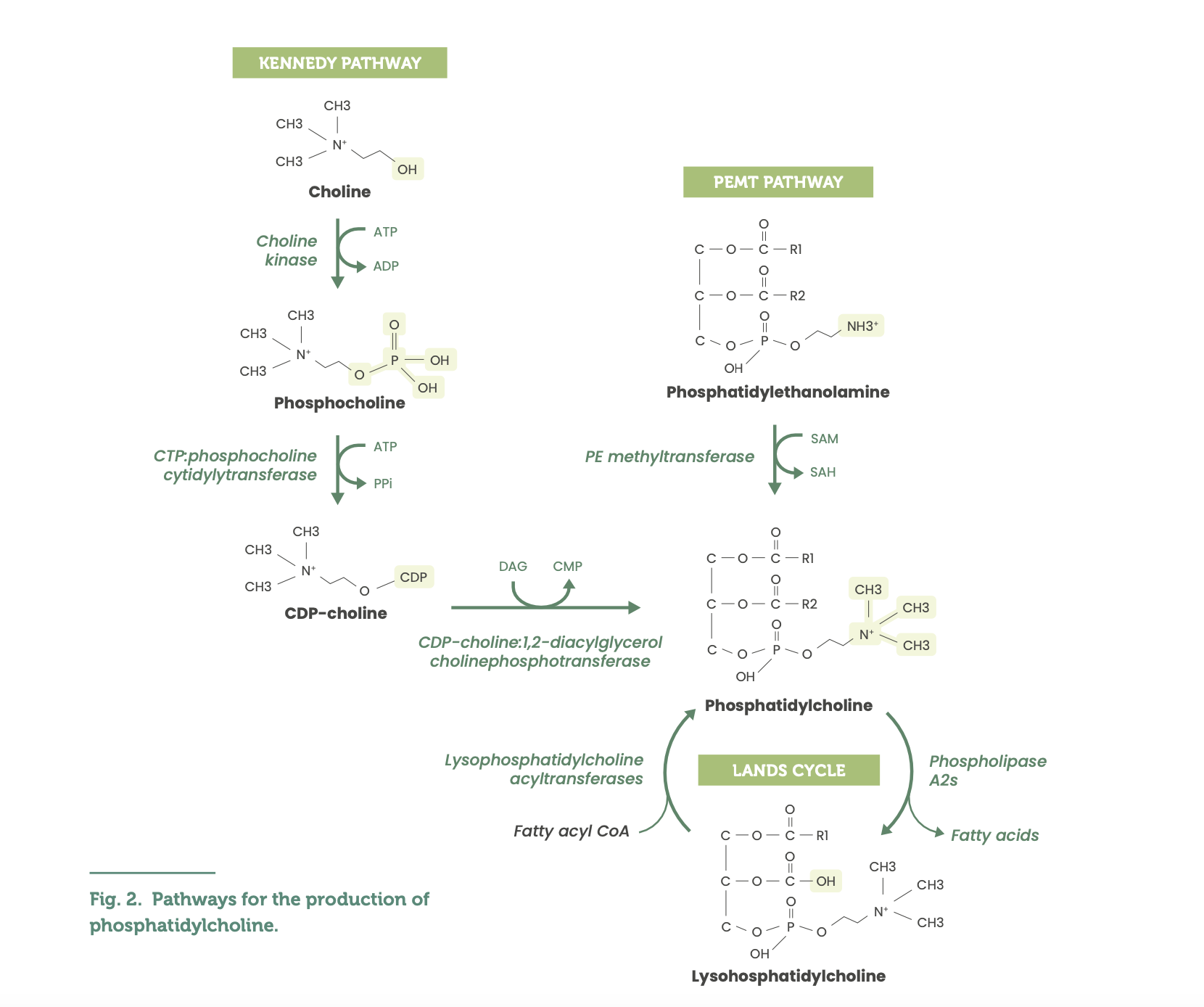
The autogenous synthesis of PC is complex, occurring via the CDP-choline pathway (also known as the Kennedy pathway), phosphatidylethanolamine N-methyltransferase (PEMT) and Lands cycle pathways. (See Fig. 2)3,4,7,8
The CDP-choline Pathway
The CDP-choline pathway, expressed by all human nucleated cells, is the main source of de novo PC synthesis.3,4,7,8 The CDP-choline pathway involves the phosphorylation of choline by ATP to phosphocholine with the assistance of choline kinase found in the cytosol.3 CTP and phosphocholine are then converted via several enzymatic processes to produce CDP-choline and diacylglycerol (DAG), generating PC.3
The PEMT Pathway
The PEMT pathway contributes a substantially smaller proportion of endogenous PC, expressed primarily in hepatic tissues, with small amounts expressed in adipose tissue.3,4,7,8 The PEMT pathway involves phosphatidylethanolamine (PE) undergoing three methylation reactions in a sequence with the methyl group donor being S-adenosylmethionine and the enzyme phosphatidylethanolamine N-methyltransferase (PEMT), producing PC.3
The Lands Cycle
The Lands cycle further emphasises the complexity of PC synthesis and its functional/biological effects, involving the remodelling of PC into subspecies (LPCATs 1-4) that are differentially distributed in body tissues including lung alveolar, immune, brain, hepatic, intestinal, adipose, and ovarian cells.8,9
Phosphatidylcholine physiological mechanisms
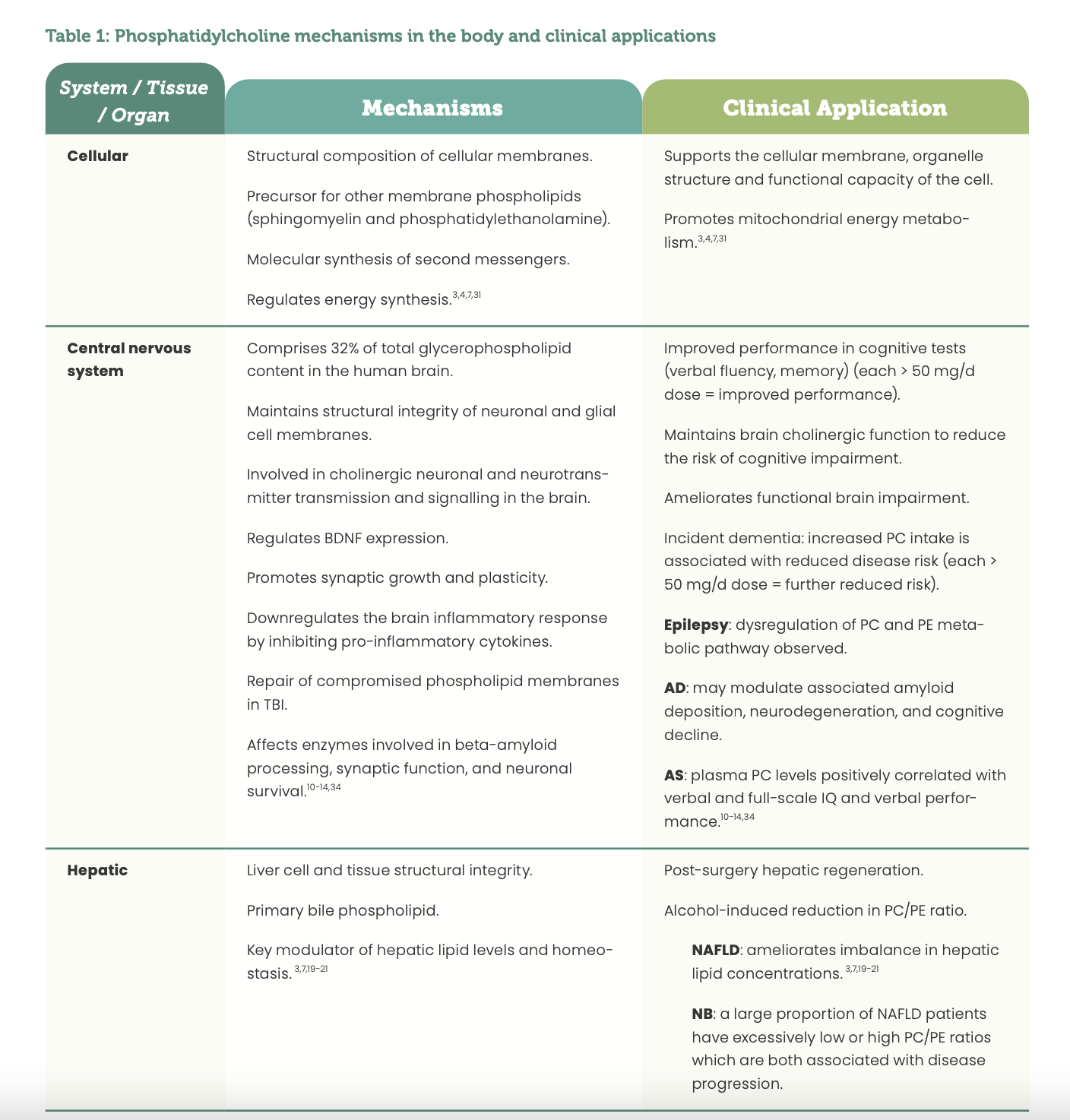
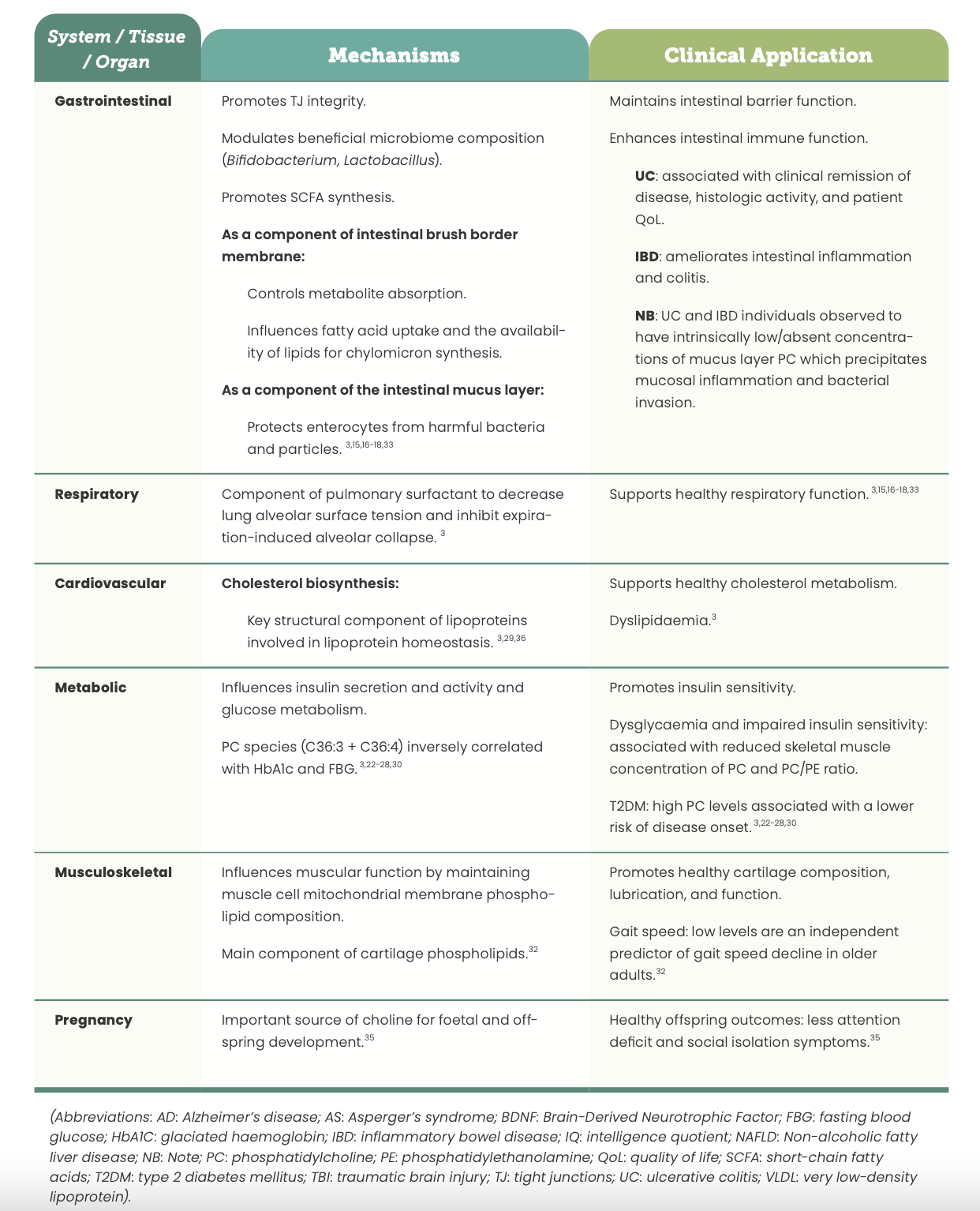
Current research regarding PC’s physiological mechanisms demonstrates its significant involvement in many central nervous system, mitochondrial, hepatic, intestinal, respiratory, and metabolic processes.3,10 Such mechanisms form the foundational basis for the clinical use and application of PC in a range of pathologies pertaining to these tissues, organs and systems as a consequence of imbalanced endogenous levels. (See Table 1)
Exogenous sources of Phosphatidylcholine
PC is available in both dietary and supplemental form, with concentrated food sources being primarily eggs, meat, fish, milk, and soy products as well as being present in smaller concentrations in potatoes and leafy greens.36-38
Several factors need to be considered when reviewing the suitability of obtaining appropriate quantities of PC from food sources alone versus supplemental sources. These factors include:
- An individuals’ normal dietary patterns, with vegetarian, vegan and low-carbohydrate diets associated with lower endogenous levels;
- Life stage (pregnancy, lactating and growth phases may increase need); and
- The presence of physiological imbalances or pathologies associated with suboptimal PC metabolism and levels (Table 1).38-40
Another significant consideration observed in a recent animal study demonstrating that compared with dietary PC intake, supplemental PC did not increase plasma levels of trimethylamine-N-oxide (TMAO), elevated levels of which are associated with adverse effects on humans in relation to colorectal cancer, cardiovascular, and kidney health.41 Further, it has been reported that as a primary source of choline, PC is absorbed more effectively compared with free choline due to reduced catabolism by the gastrointestinal microbiome.41
Physiological role of Phosphatidylcholine
Research is ongoing regarding the physiological relevance of PC and its subspecies in human health and disease. The importance of appropriate intake levels of PC to maintain normal physiological function and the impact of impaired or dysregulated metabolism and subsequent PC levels and functional effects may have in many clinical conditions have been established. Insufficient PC levels or changes to the cellular ratio of PC:PE may alter organelle energy metabolism within the cell, disrupting the function of the cell.3
Download a free PDF version of this article
References
1. Choline [Internet]. Linus Pauling Institute. 2022 [cited 28 September 2022]. Available from: https://lpi.oregonstate.edu/mic/ other-nutrients/choline
2. [Internet]. Altmedrev.com. 2022 [cited 28 September 2022]. Available from: https://altmedrev.com/wp-content/ uploads/2019/02/v7-2-150.pdf
3. van der Veen JN, Kennelly JP, Wan S, Vance JE, Vance DE, Jacobs RL. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochem Biophys Acta Biomemb 2017; 1859(9 Pt B): 1558-1572.
4. de Freitas Saito R, Nogueira de Sousa Andrade L, Bustos SO, Chammas R. Phosphatidyl-derived lipid mediators: the crosstalk between cancer cells and immune cells. Front Immunol 2022; 13: 768606.
5. LabXchange [Internet]. Labxchange.org. 2022 [cited 28 September 2022]. Available from: https://www.labxchange. org/library/pathway/lx-pathway:cdddd795-f333-4ab3-8ebb- 9ad3f80dc407/items/lx-pb:cdddd795-f333-4ab3-8ebb- 9ad3f80dc407:html:a806ca3c
6. [Internet]. Rxlist.com. 2022 [cited 28 September 2022]. Available from: https://www.rxlist.com/phosphatidylcholine/supple- ments.htm
7. da Silva RP, Eudy EJ, Denimice R. One-carbon metabolism in fatty liver disease and fibrosis: one-carbon to rule them all. J Nutr 2020, DOI: 10.1093/jn/nxaa032.
8. Wang B, Tontonoz P. Phospholipid remodelling in physiology and disease. Annu Rev Physiol 2019; 81: 165-188.
9. Wang L, Shen W, Kazachkov M, Chen G, Chen Q, Carlsson AS et al. Metabolic interactions between the Lands cycle and the Kennedy pathway of glycerolipid synthesis in Arabidopsis developing seeds. The Plant Cell 2012; 24: 4652-4669.
10. Javaid S, Farooq T, Rehman Z, Afzal A, Ashraf W, Rasool MF et al. Dynamics of choline-containing phospholipids in traumatic brain injury and associated comorbidities. Int J Mol Sci 2021; 22(21): 11313, DOI: 10.3390/ijms22211313.
11. Shea T. Choline and phosphatidylcholine may maintain cognitive performance by multiple mechanisms. The Ameri- can Journal of Clinical Nutrition 2019; 00; 1-2.
12. Martha SR, Chen KF, Lin Y, Thompson HJ. Plasma phospholipid metabolites associate with functional outcomes following mild traumatic brain injury in older adults. Biol Res Nurs 2021; 23 (1): 127-135.
13. Ajith A, Mondal S, Chattopadhyay S, Kumar A, Sthanikam Y, Chacko AG et al. Mass spectrometry imaging deciphers dysregulated lipid metabolism in the human hippocampus affected by temporal lobe epilepsy. ACS Chem Neurosci 2021; 12 (21): 4187-4194.
14. Kozielec-Oracka B, Min Y, Bhullar AS, Stasiak B, Ghebremeskel K. Plasma and red blood cell n3 fatty acids correlate positively with the WICS-R verbal and full-scale intelligence quotients and inversely with Conner’s parent-rated ADHD index t-scores in children with high functioning autism and Aspergers syndrome. Prostaglandins, Leukot Essent Fatty Acid 2022; 178; 102414.
15. Tan W, Zhang Q, Dong Z, Yan Y, Fu Y, Liu X et al. Phosphatidyl- choline ameliorates LPS-induced systemic inflammation and cognitive impairments via mediating the gut-brain axis balance. J Agric Food Chem 2020; 68(50): 14884-14895.
16. Kikut J, Konecka N, Zietek M, Kulpa D, Szczuko M. Diet supporting therapy for inflammatory bowel diseases. European Journal of Nutrition 2021; 60 (5): 2275-2291.
17. Li Q, Chen G, Zhu D, Zhang W, Qi S, Xue X et al. Effects of dietary phosphatidylcholine and sphingomyelin on DSS-induced colitis by regulating metabolism and gut microbiota in
mice. J Nutr Biochem 2022; 105: 109004, DOI: 10.1016/j.nut- bio.2022.109004.
18. Stremmel W, Vural H, Evliyaoglu O, Weiskirchen R. Delayed-re- lease phosphatidylcholine is effective for treatment of ulcerative colitis: a meta-analysis. Dig Dis 2021; 39(5): 508-515.
19. Mannisto V, Kaminska D, Karja V, Tiainen M, de Mello VD, Hanhi- neva K et al. Total liver phosphatidylcholine content associates with non-alcoholic steatohepatitis and glycine N-methyltrans- ferase expression. Liver International 2019; 39 (10): 1895-1905.
20. Ikeda Y. Mechanism of taurohyodeoxycholate-induced biliary phospholipid efflux – understanding the function of the ABCB4 enhancer for developing therapeutic agents against bile salt-induced liver injury. Yakugaku Zasshi 2020; 140(11): 1329-1334.
21. Kim YC, Seok S, Byun S, Kong B, Zhang B, Zhang Y et al. AhR and SHP regulate phosphatidylcholine and S-adenosylmethionine levels in the one-carbon cycle. Nat Commun 2018; 9(1): 540, DOI: 10.1038/s41467-018-03060-y.
22. Tremblay BL, Guenard F, Lamarche B, Perusse L, Vohl MC. Familial resemblances in human plasma metabolites are attributable to both genetic and common environmental effects. Nutr Res 2019; 61: 22-30.
23. Mahajan UV, Varma BR, Huang CW, An Y, Tanaka T, Ferrucci L et al. Blood metabolite signatures of metabolic syndrome in two cross-cultural older adult cohorts. Int J Mol Sci 2020; 21(4): 1324. DOI: 10.3390.ijms2102124.
24. Fikri AM, Smyth R, Kumar V, Al-Avadla Z, Abusnana S, Munday MR. Pre-diagnostic biomarkers of type 2 diabetes identified in the UAE’s obese national population using targeted metabolomics. Sci Rep 2020; 10 (1): 17616, DOI: 10.1038/s41598-020-73384-7.
25. Virtanen JK, Tuomainen TP, Voutilainen S. Dietary intake of choline and phosphatidylcholine and risk of type 2 diabetes in men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Eur J Nutr 2020; 59(8): 3857-3861.
26. Szczerbinski L, Golonko A, Taylor M, Puchta U, Konopka P, Pazko A, Citko A et al. Metabolomic profile of skeletal muscle and its change under a mixed-mode exercise intervention in progres- sively dysglycaemic subjects. Front Endocrinol (Lausanne) 2021; 12: 778442, DOI: 10.3389/fendo.2021.778442.
27. Prada M, Wittenbecher C, Eichelmann F, Wernitz A, Drou- in-Chartier JP, Schulze MB. Association of the odd-chain fatty acid content in lipid groups with type 2 diabetes risk: a targeted analysis of lipidomics data in the EPIC-Potsdam cohort. Clin Nutr 2021; 40(8): 4988-4999.
28. Zhang W, Randell EW, Sun G, Likhodii S, Liu M, Furey A et al. Hyperglycaemia-related advanced glycation end-products is associated with the altered phosphatidylcholine metabolism in osteoarthritis patients with diabetes. PLoS One 2017; 12(9): e0184105, DOI: 10.1371/journal.pone/0184105.
29. Paavola T, Bergmann U, Kuusisto S, Kakko S, Savolainen MJ, Salonurmi T. Distinct fatty acid compositions of HDL phospho- lipids are characteristic of metabolic syndrome and premature coronary heart disease – family study. Int J Mol Sci 2021; 22(9): 4908, DOI: 10.3390/ijims22094908.
30. Codeiro FB, Cataldi TR, de Souza BZ, Rochetti RC, Fraietta R, Labate CA, Lo Turco EG. Hyper response to ovarian stimulation affects the follicular fluid metabolomic profile of women under- going IVF similarly to polycystic ovary syndrome. Metabolomics 2018; 14(4): 51, DOI: 10.1007/s11306-018-1350-z.
31. Huang NJ, Lin YC, Lin CY, Pishesha N, Lewis CA, Freinkman E, Farquharson C et al. Enhanced phosphocholine metabolism is essential for terminal erythropoiesis. Blood 2018; 131(26): 2955- 2966.
32. Gonzalez-Freire M, Moaddel R, Sun K, Fabbri E, Zhang P, Khadeer M, Salem Jr N et al. Targeted metabolomics shows low plasma lysophosphatidylcholine 18:2 predicts greater decline of gait speed in older adults: The Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci 2019; 74(1): 62-67.
33. Thomas MS, DiBella M, Blesso CN, Malsheva O, Caudill M, Sholola M, Cooperstone JL et al. Comparison between egg intake versus choline supplementation on gut microbiota and plasma carotenoids in subjects with metabolic syndrome. Nutrients 2022; 14(6): 1179, DOI: 10.3390/nu14061179.
34. Ylilauri MP, Voutilainen S, Lonnroos E, Virtanen HEK, Tuomainen TP, Salonen JT et al. Associations of dietary choline with risk of dementia and cognitive performance: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr 2019; 110 (6): 1416-1423.
35. Ross RG, Hunter SK, Hoffman C, McCarthy L, Chambers BM, Law AJ et al. Perinatal phosphatidylcholine supplementation and early childhood behavioural problems: evidence for CHRNA27 moderation. Am J Psych 2016: 509-516.
36. Zheng Y, Li Y, Rimm EB, Hu FB, Albert CM, Rexrode KM, Manson JE et al. Dietary phosphatidylcholine and risk of all-cause and cardiovascular-specific mortality among US women and men. Am J Clin Nutr 2016; 104(1): 173-180.
37. Van Parys A, Braekke MS, Karlsson T, Vinknes KJ, Tell GS, Haugs- gjerd TR, Ueland PM et al. Assessment of dietary choline intake, contributing food items and associations with one-carbon and lipid metabolites in middle-aged and elderly adults: The Hordaland Health Study 2022; J Nutr 2022; 152(2): 513-524.
38. Murota K. Digestion and absorption of dietary glycerophos- pholipids in the small intestine: their significance as carrier molecules of choline and n-3 polyunsaturated fatty acids. Biocat Agric Biotech 2020; 26: 101633.
39. Djekic D, Shi L, Calais F, Carlsson F, Landberg R. Hyotylainen T, Frobert O. Effects of a lacto-ovo-vegetarian diet on the plasma lipidome and its association with atherosclerotic bur- den in patients with coronary artery disease – a randomised, open-label, cross-over study. Nutrients 2020; 12(11): 3586.
40. Inoue M, Senoo N, Sato T, Nishimura Y, Nakagawa T, Miyoshi N, Goda T et al. Effects of dietary carbohydrate-fat ratio on plasma phosphatidylcholine profiles in human and mouse. J Nut Biochem 2017; 50: 83-94.
41. Shirouchi B, Fukuda A, Akasaka T. Unlike glycerophosphocho- line or choline chloride, dietary phosphatidylcholine does not increase plasma trimethylamine-N-levels in Sprague-Dawl



