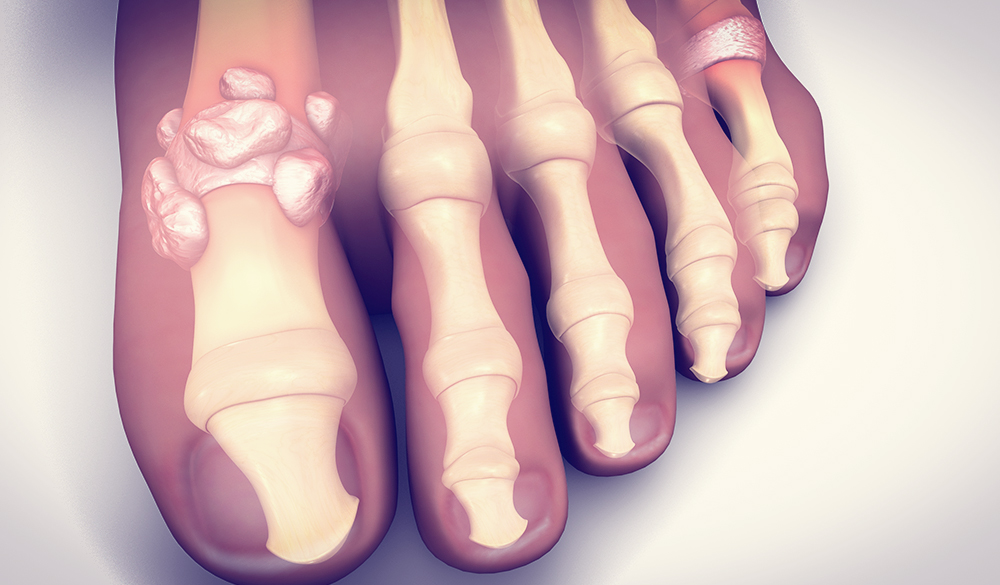
An increasingly prevalent form of inflammatory arthritis is gout.[1] Also referred to as gouty arthritis or crystal arthropathy.[2,3] The cause of gout is hyperuricaemia, defined as elevated uric acid levels in the bloodstream and tissues. Uric acid is a waste product that forms when the body breaks down purines. Normally the kidneys filter out excess uric acid,[4] however hyperuricaemia occurs when the kidneys do not adequately eliminate uric acid or if there is an over production of uric acid.[4,5]
Uric acid forms small, sharp crystals in and around the joints causing severe pain, swelling and tenderness to affected areas.[4] These crystals activate inflammatory responses that result in bone and cartilage erosions.[6] Oxidative damage is closely related to the pathogenesis of gout.[6] It has been reported that hyperuricaemia may increase the production of oxygen free radicals, promote lipid peroxidation and also up regulate pro-inflammatory mediators.[6]
Monoarticular involvement is most common in gout but multiple joints may also be involved.[7] Symptoms of severe pain, redness and swelling mostly affect the big toe (podagra),[7] foot joints especially the instep, ankles, knees, fingers and elbows.[4] Affected joints are extremely tender to touch[4] and there is a feeling that the joint is on fire.[4]
Gout attacks tend to occur suddenly at night,[4] reaching their peak within eight to twelve hours[7] and can persist for three to ten days. In some cases discomfort can continue for weeks after the initial attack.[4]
Estimates suggest that 1.5-5.2% of the Australian population is affected by gout.[8] Gout is most common in middle-aged and older men.[4,9] In women, gout typically occurs after menopause[4] as serum urate levels increase due to lower oestradiol levels.[10]
RISK FACTORS FOR GOUT
Dietary risk factors include:
- a purine-rich diet consisting of meat especially bacon, turkey, veal, venison and organ meats, and oily seafood such as anchovies, sardines, herring, mussels and scallops[11]
- excessive alcohol consumption.[4
Medical risk factors are:
- obesity as it puts additional stress on the kidneys to eliminate uric acid [4]
- certain medications including antihypertensives, low dose aspirin, cyclosporin, antirejection medications[4,12]
- medical conditions that increase uric acid levels including untreated high blood pressure, diabetes, heart disease, kidney disease and metabolic syndrome[4]
- recent surgery or trauma which can elevate uric acid levels in the blood.[4]
Other factors involve:
Gout comorbidities
Hyperuricaemia (and gout) is associated with cardiovascular and renal comorbidities,[13,14,15] including endothelial dysfunction and atherosclerosis, hypertension, coronary artery disease, diabetes, dyslipidaemia[16] and chronic kidney disease.[6,12]
Epidemiological studies also associate hyperuricaemia with the development of obesity, hypertension, insulin resistance and type 2 diabetes, all features of the metabolic syndrome.[12,17] Interestingly, early onset gout patients (first flare up before 40 years of age) develop earlier metabolic disorders and slightly more severe joint involvement than common gout patients who have their first flare up after 40 years.[18]
In cases where gout has been left untreated, attacks are likely to recur and can increase in frequency.4 Patients can develop tophi (small lumps which form under the skin4), joint damage4 and kidney stones.4 The condition in some cases can resemble rheumatoid arthritis.[7]
MANAGING GOUT
Despite the accessibility of effective treatments for gout, the condition is commonly poorly managed. Aspects of treating gout include inhibition of uric acid overproduction, managing inflammation and oxidative stress.[6] Reduction and maintenance of serum uric acid levels to less than 6.0mg/dL is generally the treatment goal in the management of hyperuricaemia.[13]
Poor patient compliance to treatment[1,19] is attributed to an increased risk of acute flares after commencing urate-lowering therapy (ULT).[20] In a South Australian population-based study just over 50% of the respondents with gout who commenced allopurinol continued to take it as prescribed.[8]
ULT includes uricosuric drugs, which enhance renal uric acid excretion, and xanthine oxidase (XO) enzyme inhibitors such as allopurinol and febuxostat.[13,6] XO inhibitors reduce the uric acid contents of plasma and urine and decrease the deposition of uric acid in soft tissue.[6] XO is a hepatic enzyme that is generated from xanthine dehydrogenase during oxidative stress and inflammatory processes. The enzyme oxidises hypoxanthine to xanthine and then to uric acid.[6]
The clinical use of allopurinol is limited because of adverse effects such as hepatotoxicity, nephrotoxicity and hypersensitivity reactions.[6] Febuxostat is prescribed secondary to allopurinol for those patients who do not tolerate allopurinol.[8] Febuxostat can cause liver function abnormalities, nausea, joint pain and rash.[13]
Showing potential in gout management are celery, sour cherry and baheda. Various studies show that they are well tolerated, efficacious and have fewer or no side effects compared to traditional ULT.
Apium graveolens (celery)
Traditionally used to treat rheumatism and cardiovascular disorders, celery has a long history of use in Ayurveda and Unani medicine.[6] Celery may reduce the degeneration of body joints that normally occurs with age and can also ease joint discomfort that occurs due to inflammation.[21]
The uricosuric action[21] and anti-gout activity of celery is due to phthalide derivatives, phenolic compounds and tannins.[6] Celery also has powerful antioxidant characteristics due to compounds such as caffeic acid, p-coumaric acid, ferulic acid, apigenin, luteolin, tannin, saponin and kaempferol.[22]
Apigenin a flavonoid of celery has hyperuricaemic and XO inhibitory activity. It has been reported that apigenin could interact with the XO active site and is comparable to allopurinol in vitro.[6,23]
Celery luteolin reversibly inhibits XO in a competitive manner, by interacting with the primary amino acid residues located within the active site pocket of XO. Kaempferol in celery has been reported to reversibly inhibit XO by occupying the catalytic centre of the enzyme. Additionally, luteolin has been shown to exhibit a strong synergistic effect with kaempferol.[6]
Prunus cerasus (sour cherry)
Reports from clinical populations have indicated a reduced incidence of gout attacks following regular consumption of tart cherry juice concentrate across a four-month period.[20,24] Cherry products may reduce the pain associated with gout and some patients consume cherries in various forms to reduce the effects of gout attacks.[24,25,26]
Small experimental studies in human and animal subjects have demonstrated that the anthocyanin of cherries lowers serum uric acid levels by inhibiting XO activity.[24,26,27] Investigators also speculate that cherries may lower urate levels by increasing the glomerular filtration rate or reducing tubular reabsorption.[24]
In a large study of prospectively recruited patients with pre-existing gout, fresh cherry intake was associated with a 35% lower risk of recurrent gout attacks, and cherry extracts showed a similar inverse association. Interestingly, when cherry intake was combined with allopurinol, the risk of gout attacks was 75% lower than over the period without either exposure.[24,26]
The high levels of anthocyanin and vitamin C in cherries are responsible for the anti-inflammatory and antioxidant properties of the fruit[24,26,27] either through inhibiting cyclooxygenase (COX) activity or via scavenging nitric oxide radicals.[20,24]
Terminalia bellirica (baheda)
Baheda is a component of Triphala, an Ayurvedic formulation commonly used in India. Baheda has been shown to have XO enzyme inhibitory activity and superoxide radical scavenging activity.[13] In vitro studies comparing the XO inhibitory activity of baheda and allopurinol concluded that it was as efficacious as allopurinol.[13]
Baheda also possesses anti-inflammatory action possibly due to inhibition of inducible nitric oxide synthase.[13]
These features of celery, sour cherry and baheda make them appealing alternatives for the prophylactic management of hyperuricaemia and gout.
References
- Rogenmoser S, Arnold MH. Chronic gout: barriers to effective management. Aust J Gen Pract 2018;47(6):351-356. [Abstract]
- Fazel M, Merola JF, Kurtzman DJB. Inflammatory arthritis and crystal arthropathy: current concepts of skin and systemic manifestations. Clin Dermatol 2018;36(4):533-550. [Abstract]
- Sidari A, Hill E. Diagnosis and treatment of gout and pseudogout for everyday practice. Prim Care 2018;45(2):213-236.[Abstract]
- Gout. Viewed 1 August 2018, [Source]
- What causes gout? Viewed 4 August 2018, [Source]
- Dolati K, Rakhshandeh H, Golestani M, et al. Inhibitory effects of Apium graveolens on xanthine oxidase activity and serum uric acid levels in hyperuricemic mice. Prev Nutr Food Sci 2018;23(2):127-133. [Abstract]
- Gout and pseudogout. [Source]
- Pisaniello HL, Lester S, Gonzalez-Chica D, et al. Gout prevalence and predictors of urate-lowering therapy use: results from a population-based study. Arthritis Res Ther 2018;20:143. [Abstract]
- Australian Institute of Health and Welfare. What is gout? [Source]
- Jansen Dirken-Heukensfeldt KJM, Teunissen TAM, van de Lisdonk EH, et al. Clinical features of women with gout arthritis. A systemic review. Clin Rheumatol 2010;29:575-582.[Abstract]
- Safe foods for gout. [Source]
- Bardin T, Richette P. Impact of comorbidities on gout and hyperuricaemia: an update on prevalence and treatment options. BMC Medicine 2017;15:123.[Abstract]
- Usharani P, Nutalapati C, Pokuri VK, et al. A randomised, double-blind, placebo-, and positive-controlled clinical pilot study to evaluate the efficacy and tolerability of standardized aqueous extracts of Terminalia chebula and Terminalia bellerica in subjects with hyperuricemia. Clinical Pharmacology: Advances and Applications 2016;8:51-59.[Abstract]
- Murray K, Burkard T. Hyperuricemia, gout and cardiovascular diseases. Ther Umsch 2016;73(3):141-146.[Abstract]
- Janssens HJ, Arts PG, Schalk BW, et al. Gout and rheumatoid arthritis, both to keep in mind in cardiovascular risk management: A primary care retrospective cohort study. Joint Bone Spine 2017;84(1):59-64.[Abstract]
- Zhang QB, Zhu D, Wen Z, et al. High levels of serum uric acid, cystain C and lipids concentration and their clinical significance in primary gouty arthritis patients. Curr Rheumatol Rev 2018;doi:10.2174/1573397114666180705095625. [Abstract]
- Marin M, Maalouf NM. Effects of pharmacological reversal of hyperuricemia on features of the metabolic syndrome in patients with gouty arthritis. J Investig Med 2018;pii:jim-2018-000728.[Abstract]
- Pascart T, Norberciak L, Ea HK, et al. GOSPEL 4 - Patients with early onset gout develop earlier severe joint involvement and metabolic comorbid conditions. Arthritis Care Res (Hoboken). 2018;19:doi:10.1002/acr.23706.[Abstract]
- Counsell AB, Nguyen AD, Baysari MT, et al. Exploring current and potential roles of Australian community pharmacists in gout management: a qualitative study. BMC Fam Pract. 2018;19(1):54.[Abstract]
- Schlesinger N, Rabinowitz R, Schlesinger M. Pilot studies of cherry juice concentrate for gout flare prophylaxis. J Arthritis 2012;1:1.[Abstract]
- Asif HM, Akram M, Usmanghani K, et al. Monograph of Apium graveolens Linn. J Med Plants Res 2011;5(8):1494-1496. [Abstract]
- Kooti W, Daraei N. A review of the antioxidant activity of celery (Apium graveolens L). JEBCAM 2017;22(4):1029-1034. [Abstract]
- Lin CM, Chen CS, Chen CT, et al. Molecular modeling of flavonoids that inhibits xanthine oxidase. Biochem Biophys Res Commun 2002;294(1):167-72.[Abstract]
- Zhang Y, Neogi T, Chen C et al. Cherry consumption and the risk of recurrent gout attacks. Arthritis Rheum 2012;64(12): 4004-4011. [Abstract]
- Bell PG, Gaze DC, Davison GW, et al. Montmorency tart cherry (Prunus cerasus L.) concentrate lowers uric acid, independent of plasma cyaniding-3-O-glucosiderutinoside. J Funct Foods II 2014;11:82-90. [Abstract]
- Kelley DS, Adkins Y, Laugero KD. A review of the health benefits of cherries. Nutrients 2018;10:368.[Abstract]
- Haidari F, Mohammad Shahi M, Keshavarz SA, et al. Inhibitory effects of tart cherry (Prunus cerasus) juice on xanthine oxidoreductase activity and its hypouricemic and antioxidant effects on rats. Malays J Nutr 2009;(1):53-64.[Abstract]
DISCLAIMER:
The information provided on FX Medicine is for educational and informational purposes only. The information provided on this site is not, nor is it intended to be, a substitute for professional advice or care. Please seek the advice of a qualified health care professional in the event something you have read here raises questions or concerns regarding your health.