Coeliac disease (CD) is an autoimmune disorder triggered by the ingestion of gluten, a major protein in wheat, or of related proteins in other grains such as barley and rye. Research into the root cause indicates that the disorder develops when a person exposed to gluten also has a genetic susceptibility to CD and an unusually permeable intestinal wall.
Identification of gluten as the trigger occurred after World War II, when Dutch paediatrician Willem-Karel Dicke noticed that a war-related shortage of bread in the Netherlands led to a significant drop in the death rate among children affected by CD - from greater than 35% to essentially zero. He also reported that when wheat was once again available after the conflict, the mortality rate soared to previous levels.[1]
In CD, it is now clear that gluten itself prompts exaggerated zonulin secretion (certainly in part because of the patient’s genetic makeup). But this is not unique to just CD. In fact, a search of the medical literature reveals many autoimmune diseases, including type 1 diabetes, multiple sclerosis, rheumatoid arthritis and inflammatory bowel diseases, all have as a common denominator aberrant intestinal permeability. In many of these diseases, the increased permeability is caused by abnormally high levels of zonulin.[1]
A growing body of evidence suggests that virtually the same trio of factors underpins most, and possibly all, autoimmune diseases. Dr Alessio Fasano looks at CD as a potential model for autoimmune conditions generally and the three underlying factors they share are:[1]
- an environmental trigger
- a genetic predisposition
- a leaky small intestine.
He suspects that, whilst the same basic triad contributes to other autoimmune diseases, each disorder will have its own triggers and genetic components. This does raise the distinct possibility that new treatments for CD may also ameliorate other autoimmune-based conditions.
As to why some autoimmune conditions may be triggered earlier in life, and others may take some time, Dr Fasano suggests this has to do with gut microflora.
These microbes, collectively known as the microbiome, may differ from person to person and from one population to another, even varying in the same individual as life progresses. Apparently they can also influence which genes in their hosts are active at any given time. Hence, a person whose immune system has managed to tolerate gluten for many years might suddenly lose tolerance if the microbiome changes in a way that causes formerly quiet susceptibility genes to become active. If this idea is correct, coeliac disease might one day be prevented or treated by ingestion of selected helpful microbes, or ‘probiotics’. - Dr Alessio Fasano
Removing provocative dietary agents, such as wheat, is helpful to reduce triggering a genetic propensity for autoimmune disease. However, ensuring gastrointestinal integrity also goes a long way to supporting the gastrointestinal associated lymphoid tissue (GALT) and limiting the paracellular passage of macromolecules.
Mucins are highly glycosylated macromolecules, forming the first barrier between the contents of the gut and epithelial cells. This barrier provides protection for the epithelial cells from direct contact with commensal bacteria and their elements. Changes in either the composition or amount of mucus may lead to inflammatory responses[2] and thus supporting appropriate production is important.
The GALT serves as a containment system preventing potentially harmful intestinal antigens from reaching the systemic circulation and induces systemic tolerance against luminal antigens by a process that involves secretory IgA production and the induction of regulatory T cells.
Secretory IgA is one of the main humoral defense mechanisms ensuring the proper functioning of the mucosal surface barrier. It prevents the adherence of bacteria to mucosal surfaces and the penetration of antigens into the internal environment of the host by specific and non-specific mechanisms.[3,4] However, in persons with selective IgA deficiency, the mucosal barrier is deficient and more permeable to immunogens and allergens. In turn, supporting secretory IgA production is also a key strategy.
And finally, up-regulation of zonulin activity in genetically susceptible individuals leads to autoimmune diseases.[5] Zonulin expression is augmented in autoimmune conditions associated with tight junction (TJ) dysfunction, including CD.[6,7] It appears that attenuating this overzealous response may be integral to restoring immunological tolerance.
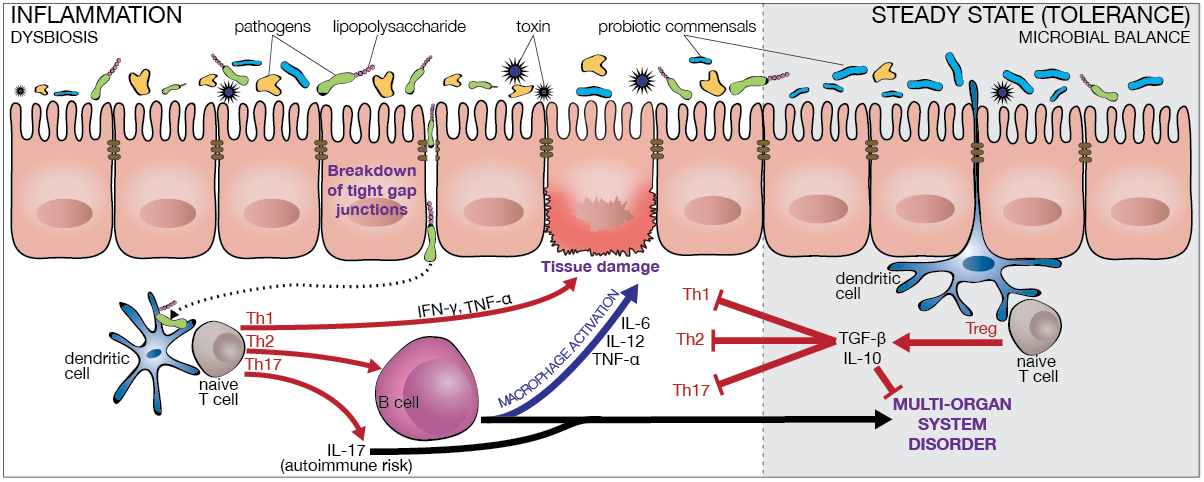
This balance between immunity and tolerance is essential for a healthy intestine, and abnormal or inappropriate immune responses can certainly result in inflammatory pathologies.
Dendritic cells are the main cells that present antigens to the adaptive arm of the mucosal immune system.[8] A mucosal immune response, either one of tolerance or stimulation, depends on the partaking of different populations of dendritic cells responsible for the activation of regulatory T cell subpopulations,[9] thereby supporting the role for dendritic cells in coupling innate and adaptive immune responses that affect intestinal permeability.
Activation of regulatory T cells that inhibit the immune response and induce mucosal tolerance is dependent on the production of IL-10 and transforming growth factor-beta10 and this is heavily influenced by commensal microorganisms. The maturation of dendritic cells is dependent on inducement by pathogenic organisms and this then brings about the activation of effector T cells crucial for clearing infections and the prevention of subsequent infections with the same or related bacteria.
In the event our in-built protection system fails, or responds in an overly aggressive manner, an unchecked inflammatory response can have serious consequences.
Therefore, strategies for addressing autoimmune conditions would do well to include removing provocative dietary antigens, strengthening the gastrointestinal barrier (structurally and functionally), and driving anti-inflammatory processes by modulating zonulin activity and improving the microbiome.
References
- Fasano A. Surprises from celiac disease. Scientific American 2009:32-39.
- Linden SK, Sutton P, Karlsson NG, et al. Mucins in the mucosal barrier to infection. Mucosal Immunology 2008;1(3):183-197. [Abstract]
- Mestecky J, Russell MW, Elson CO. Intestinal IgA: novel views on its function in the defence of the largest mucosal surface. Gut 1999;44(1):2-5. [Full text]
- Brandtzaeg P. Update on mucosal immunoglobulin A in gastrointestinal disease. Curr Opin Gastroenterol 2010;26(6):554-563. [Abstract]
- Fasano A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann N Y Acad Sci 2012;1258:25-33. [Full text]
- Fasano A, Not T, Wang W, et al. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet 2000;355(9214):1518-1519. [Abstract]
- Sapone A, de Magistris L, Pietzak M, et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes 2006;55(5):1443-1449. [Full text]
- Rescigno M, di Sabatino A. Dendritic cells in intestinal homeostasis and disease. J Clin Investig 2009;119(9):2441-2450. [Full text]
- Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nature Rev Immunol 2008;8(6):435-446. [Full text]
- Barnes MJ, Powrie F. Regulatory T cells reinforce intestinal homeostasis. Immunity 2009;31(3):401-411. [Full text]
DISCLAIMER:
The information provided on FX Medicine is for educational and informational purposes only. The information provided on this site is not, nor is it intended to be, a substitute for professional advice or care. Please seek the advice of a qualified health care professional in the event something you have read here raises questions or concerns regarding your health.