Georgia Marrion ● 7 min read
Part 1: The interconnection between regulation of glucose metabolism and female reproduction
Macronutrients are essential for both energy metabolism and as biological signalling components in the body. In women, the interconnection between energy metabolism and reproductive health and function is significant, yet complex.[1] The health impact of one such macronutrient group, carbohydrates, particularly the intake of simple sugars, has been the subject of much interest, research and demonisation over recent years.
But what does the evidence demonstrate regarding the impact of sugar intake on female reproductive health?
This two-part article series reviews such evidence in premenopausal women, in the absence of overt or diagnosed metabolic pathologies, and its relevance from a clinical perspective.
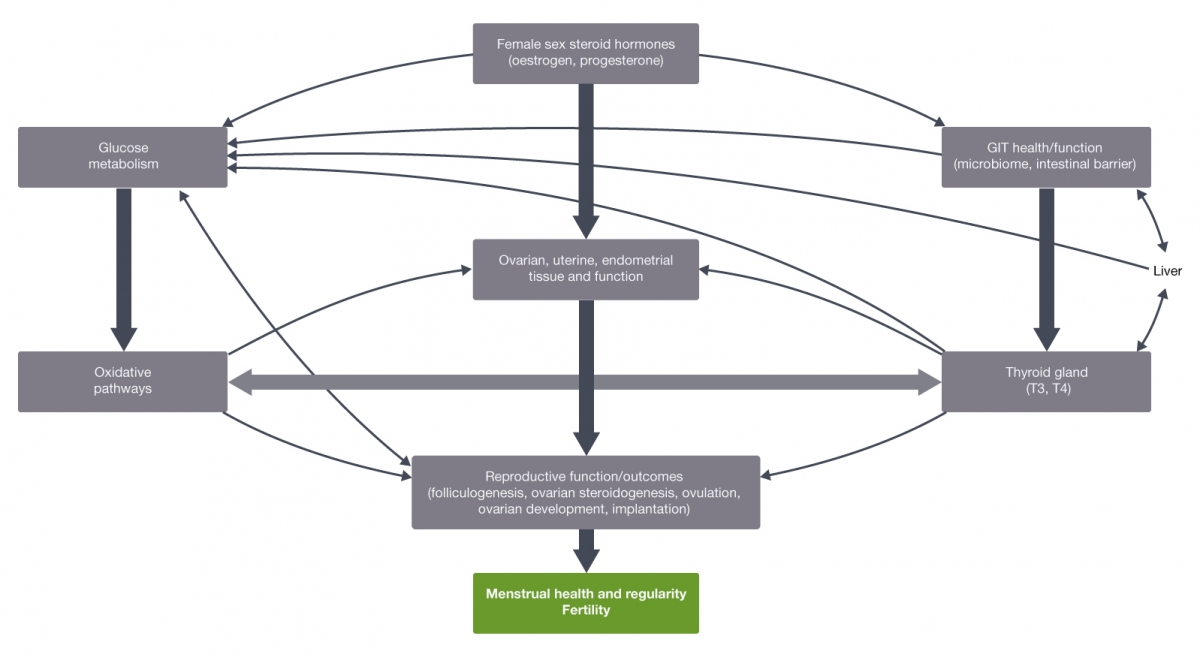
When reviewing the potential impact of sugar intake on female reproductive health, factors to be considered include the various body systems, organs and tissues involved in (either or both) glucose metabolism and reproductive function, and the bidirectional functional relationships between many of them (covered in Part 1). Following on from these interconnections, how sugar can impact these body systems, organs and tissues, both individually and via their functional interconnection, and the clinical relevance of these effects also needs to be considered. This will be covered in Part 2.
Reproductive function
Sex steroid hormones, the thyroid gland and hormones, the gastrointestinal tract and the oxidative metabolic pathways function synergistically to regulate female reproductive physiology.
The biological activity of oestrogen is modulated via the oestrogen receptors alpha and beta (ERα & ERβ), with their endogenous actions extending beyond reproductive function to include their involvement in glucose metabolism,[2-4] gastrointestinal health and function,[5-7] and oxidative metabolic pathways.[8]
The sex steroid hormones, oestrogen (particularly 17β oestradiol (E2), the predominant endogenous oestrogen) and progesterone, are intimately involved in regulating the menstrual cycle and promoting an overall environment conducive to fertilisation, implantation and early embryonic development.[9,10]
In premenopausal women, oestrogen synthesis from the developing ovarian follicles during the follicular phase increases until ovulation. Following ovulation during the luteal phase, corpus luteum-derived progesterone levels increase until the onset of menstruation.[9] The bioavailability and bioactivity of oestrogens is regulated by the hepatic-synthesised glycoprotein sex hormone binding globulin (SHBG), with bound oestrogens being biologically inactive.[11]
Along with sex steroid hormones, female reproductive function is also significantly influenced by the thyroid gland and hormones (thyroxine (T4) and triiodothyronine (T3)) via many molecular mechanisms that regulate the menstrual cycle (endometrial thickness, folliculogenesis, oocyte development, luteogenesis and ovulation), fertility (fertilisation, implantation) and embryonic development.[12] The normal functioning of these processes is mediated by direct, thyroid hormone receptor-mediated action (and tissue responsiveness) on oocyte, ovarian surface epithelium, endometrium, placenta and foetal tissues.[12,13] Thyroid hormones can also affect the hepatic production of SHBG and therefore circulating levels and functional effects of E2 in the body.[12,14]
RELATED ARTICLE - Supplementing a healthy pregnancy by Rebecca Guild
The close interrelationship between sex steroid hormones and the thyroid is largely a consequence of their dual regulation by the hypothalamus and pituitary glands.[12] This is also demonstrated by associations between hypo- and hyperthyroid hormone levels and altered E2 concentrations. This is partly due to low SHBG levels, which produces lower total levels of circulating E2. This leads to → impaired follicular maturation → reduced ovulation → (which clinically can present as) abnormal menstrual patterns and reduced fertility.[12,14,15]
The gastrointestinal tract and hepatic influence on reproductive health and function is also significant via a range of processes. Hepatic mechanisms involve regulating cholesterol-derived and enzyme (aromatase) steroid hormone synthesis and the metabolism of oestrogens and oestrogen metabolites.[16] Oestrogen metabolism occurs primarily via phase 1 conjugation (hydroxylation by cytochrome P450 CYP 2B1,1A and 3A) to produce oestradiol, oestrone and their respective metabolites that can be subsequently metabolised through the sulfation and glucuronidation pathways (and excreted into bile, urine or passed into intestines), and via methylation (catechol-O-methyltransferase (COMT)).[9,16,17] Other hepatic mechanisms that influence reproductive function include the synthesis of the steroid carrier proteins including SHBG and sulfation of T4 and T3.[16]
The involvement of the gastrointestinal tract in reproductive function is largely through a range of microbiome-derived mechanisms. These mechanisms include intestinal metabolism (hydrolysis and deconjugation) of oestrogens via beta-glucuronidase bacterial enzyme capacity;[9,18] altering the expression of functional reproductive-associated genes (i.e. aromatase cytochrome p19, alpha isoform of the oestrogen receptor, luteinising, hydroxysteroid dehydrogenase and membrane progesterone receptors alpha (α) and beta (β));[19] and the synthesis and intestinal translocation of lipopolysaccharides (LPS) which can interfere with oestradiol and progesterone levels and their functional effects.[19]
The microbiome can also synthesise thyroid hormone, producing approximately 20% of peripheral bioactive T3.[19] Following intestinal bacterial metabolism, thyroid hormones and oestrogen are reabsorbed into the bloodstream via entero-hepatic circulation.[9] Consequently, the gastrointestinal tract influences endogenous sex steroid and thyroid hormone concentrations and therefore their functional effects on reproductive organs, tissues and processes (e.g. menstrual cycles, ovarian morphology).[6]
This relationship between sex steroid hormones and gastrointestinal function is bidirectional, with oestrogen observed to modulate gastrointestinal microbiome composition, and both oestrogen and progesterone influencing gastrointestinal epithelial tight junction expression and permeability.[18,20,21]
Oxidative metabolism can also modify reproductive function, with a healthy ratio of reactive oxygen species (ROS) and endogenous antioxidant components required for the normal functioning of folliculogenesis, ovarian steroidogenesis, ovulation, embryo development and implantation. By adversely impacting these biological processes, an imbalanced antioxidant/prooxidant ratio towards a ROS-predominant environment, particularly in ovarian and uterine tissue, may contribute to infertility, endometriosis, anovulation and impaired oocyte quality pathogenesis.[8,22]
Glucose metabolism
The synergistic relationship between sex steroid hormones, the thyroid gland and the gastrointestinal tract in regulating female reproductive function is also observed in their interdependent influence on glucose metabolism.
The female sex hormones influence several parts of the glucose metabolic pathway. Oestrogen-regulated mechanisms include: modulating insulin synthesis, secretion and tissue insulin responsiveness; pancreatic beta-cell survival, numbers, health and function; glucose uptake (insulin- and contraction-stimulated) and whole body glucose kinetics.[1-4,23,24]
Oestrogen also regulates hepatic glucose metabolic processes including insulin clearance, gluconeogenesis and glycogen synthesis[25] and, further, modulates muscle and adipose tissue glucose utilisation.[10] Conversely, progesterone antagonises oestrogen-regulated skeletal muscle glucose uptake and metabolism.[26,27]
The influence of sex steroid hormones on glucose homeostasis can also be observed by variations in glucose utilisation in the follicular phase (higher oestrogen/lower progesterone → higher glucose utilisation) and luteal phase (higher progesterone/lower oestrogen → reduced insulin sensitivity and glucose utilisation). Premenopausal women have increased systemic insulin sensitivity and glucose utilisation compared with men. The use of oral contraceptives is associated with reduced insulin sensitivity and low oestrogen levels are associated with increased predisposition to metabolic dysfunction (e.g. type 2 diabetes mellitus).[16,27]
With a primary function of the thyroid gland being the regulation of energy metabolism, it is naturally closely involved in carbohydrate (glucose) homeostasis and metabolism. Specifically, thyroid hormones regulate insulin secretion, activity, sensitivity and clearance, glucose utilisation and gluconeogenesis.[29-32] Consequently, suboptimal thyroid function (hypo- or hyperactivity) can interfere with glucose metabolic processes and consequently endogenous glucose concentrations.
The gastrointestinal tract and liver also influence glucose homeostasis. The microbiome can modulate glucose metabolism, beta cell function and gastrointestinal barrier integrity, which in-turn can affect insulin sensitivity via the presence (or absence) of excessive oxidative stress and inflammation induced by suboptimal intestinal barrier function.[19,32]
Hepatic influence on carbohydrate metabolism and glucose homeostasis involves several mechanisms including the management of fasting plasma glucose concentrations (i.e. via gluconeogenesis and glyconeogenesis), and SHBG regulation of glucose transporters independent of its role as a transporter of sex hormones (i.e. elevated levels of SHBG are associated with reduced risk of type 2 diabetes mellitus, while conversely low levels have been correlated with elevated HbA1c levels).[16,33,34]
Further evidence of the interconnection between these systems, organs and tissues, glucose metabolism and female reproductive can be observed in the ‘monitoring’ role of hepatic ERα in menstruation cycle-induced variations in circulating E2 concentrations, and the subsequent modification in hepatic energy production as per requirements during each menstrual phase.[16] Microbiome composition differences are observed in women with endometriosis, with higher concentrations of bacteria with beta-glucuronidase enzyme capacity. This leads to increased endogenous circulating oestrogen metabolites, which is one driving factor for endometriosis.[18] Associations are also observed between insulin resistance and the severity of menstrual irregularities (e.g. oligomenorrhoea and amenorrhoea).[35,36]
The complexity of these functional interconnections, between the various body systems, organs and tissues involved in glucose metabolism and female reproductive function, is essential to be taken into account when considering the variable (both within and between females), known, and potential mechanistic effects and health outcomes of excessive dietary sugar intake on female reproductive health.
REFERENCES
- Fontana R, Della Torre S. The deep correlation between energy metabolism and reproduction: a view on the effects of nutrition for women fertility. Nutrients 2016; 8: 87 [Full Text]
- Guillaume M, Montagner A, Fontaine C, et al. Nuclear and membrane actions of estrogen receptor alpha: contribution to the regulation to energy and glucose homeostasis. Adv Exp Med Biol 2017; 1043: 401-426 [Abstract]
- Ma W, Chen X, Cerne R, et al.. Catechol estrogens stimulate insulin secretion in pancreatic beta-cells via activation of the transient receptor potential A1 (TRPA1) channel. J Biol Chem 2019 Feb 22; 294 (8): 2935-2946 [Full Text]
- Qian SW, Liu Y, Wang J, et al. BMP4 cross-talks with oestrogen/ER signalling to regulate adiposity and glucose metabolism in females. EBioMedicine. 2016 Sep; 11: 91-100 [Full Text]
- Vom Steeg LG, Klein SL. Sex steroid mediate bidirectional interactions between hosts and microbes. Hormones and behaviour 2017: 88: 45-51 [Full Text]
- Zhang F, Ma T, Cui P, et al. Diversity of the gut microbiota in dihydrotestosterone-induced PCOS rats and the pharmacologic effects of Diane-35, probiotics and berberine. Front Microbiol 2019 Feb 8 [Full Text]
- Org E, Mehrabian M, Parks BW, et al. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes 2016; 7 (4) [Full Text]
- Sadowska J, Dudzinkska W, Skotnicka E, et al. The impact of a diet containing sucrose and systematically repeated starvation on the oxidative status of the uterus and ovary of rats. Nutrients 2019; 11 (7): 1544. [Full Text]
- Trickey R. Women, hormones and the menstrual cycle. Trickey Enterprises Pty Ltd. 2011: Melbourne.
- Barreto-Andrade JN, de Fatima LA, Campello RS, et al. Oestrogen receptor 1 (ESR1) enhances Sic2a4/GLUT4 expression by a SP1 cooperative mechanism. Int J Med Sci 2018 Aug 10: 15 (12): 1320-1328 [Full Text]
- Deswal R, Yadav A, Dang AS, Sex hormone binding globulin – an important biomarker for predicting PCOS risk: a systematic review and meta-analysis. Syst Biol Reprod Med 2018 Feb; 64 (1): 12-24 [Abstract]
- Silva JF, Ocarino NM, Serakides R. Thyroid hormones and female reproduction. Biology Reproduction 2018; 99 (5): 907-921 [Abstract]
- Dosiou C. Thyroid and fertility: recent advances. Thyroid 2020 Feb: doi: 10.1089/thy.2019.0382 [Full Text]
- Saran S, Gupta BS, Philip R, et al. Effect of hypothyroidism on female reproductive hormones. Indian J Endocrinol 2016 Jan-Feb; 20 (1): 108-113 [Full Text]
- Liu J, Guo M, Hu X, Weng X, et al. Effects of thyroid dysfunction on reproductive hormones in female rats. Chinese J Physiol 2018; 61 (3): 152-162 [Abstract]
- Soria-Jasso LE, Carino-Cortes R, Munoz-Perez VM, et al. Beneficial and deleterious effects of female se hormones, oral contraceptives, and phytoestrogens by immunodulation on the liver. Int J Mol Sci 2019; 20: 4694 [Full Text]
- Whirl-Carrillo M, McDonagh EM, Hebert JM, et al. Pharmacogenomics knowledge for personalised medicine. Clin Pharm Ther Oct 2012; 92 (4): 414-7 [Full Text]
- Baker JM, Al-Nakkash L, Herbst-Kralovetz MM. Estrogen-gut microbiome axis: physiological and clinical implications. Maturitas 2017; 103: 45-53 [Abstract]
- Kunc M, Gabrych A, Witkowski JM. Microbiome impact on metabolism and function of sex, thyroid, growth and parathyroid hormones. Acta Biochmica Polonica 2016; 63 (2): 189-201 [Abstract]
- Roomruangwong C, Carvalho AF, Geffard M, et al. The menstrual cycle may not be limited to the endometrium but may also impact permeability. Acta Neuropsychiatrica 2019; 31: 294-304 [Abstract]
- Zhou Z, Zhang L, Ding M, et al. Oestrogen decreases tight junction protein ZO-1 expression in human primary gut tissues. Clin Immunol 2017 Oct; 183: 174-180 [Full Text]
- Diamanti-Kandarakis E, Papalou O, Kandaraki EA, et al. Mechanisms in Endocrinology: Nutrition as a mediator of oxidative stress in metabolic and reproductive disorders in women. Eur J Endocrinol 2017; 176: R79-R99 [Abstract]
- Mauvais-Jarvis F, Le May C, Tiano JP, et al. The role of estrogens in pancreatic islet physiopathology. Adv. Exp Med Biol 2017; 1043: 385-399 [Abstract]
- Rehrer NJ, McLay-Cooke RT, Sims ST. Sex hormones, exercise and women: Chapter 6 - nutritional strategies and sex hormone interactions in women. 2017. Springer International: Switzerland.
- Shen M, Shi H. Sex hormones and their receptors regulate liver energy homeostasis. Int J Endocrinol 2015; Article ID: 294278 [Full Text]
- Varlamov O. Western-style diet, sex steroids and metabolism. Biochemica et Biophysica Acta 2017; 1863: 1147-1155 [Full Text]
- Varlamov O, Bethea CL, Roberts CT. Sex-specific differences in lipid and glucose metabolism. Front Endocrinol (Lausanne) 2014; 5: 241 [Full Text]
- Chen RH, Chen HY, Man KM, et al. Thyroid diseases increased the risk of type 2 diabetes mellitus. Medicine 2019; 98: 20 [Full Text]
- Jang J, Kim Y, Shin J, et al. Association between thyroid hormones and the components of metabolic syndrome. BMC Endocrine Disorders 2018 May; 18: 29 [Full Text]
- McAninch EA, Bianco AC. Thyroid hormone signalling in energy homeostasis and energy metabolism. Ann N Y Acad Sci 2014 Apr; 1311: 77-87 [Full Text]
- Mullar R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev 2014: 94; 355-382 [Full Text]
- Lerner A, Jeremias P, Matthias T. Gut-thyroid axis and celiac disease. Endocr Connect 2017 May; 6 (4): R52-R58 [Full Text]
- Feng C, Jin Z, Sun L, et al. Endogenous SHBG levels correlate with that of glucose transporters in insulin resistance model cells. Mol Biol Rep 2019 Oct; 46 (5): 4953-4965 [Abstract]
- Sofer Y, Nevo N, Vech M, et al. Human sex hormone-binding globulin does not provide metabolic protection against diet-induced obesity and dysglycaemia in mice. Endocrine Connections 2018; 7: 91-96. [Full Text]
- Rostami Dovom M, Ramezani Tehrani F, Djalalinia S, et al. Menstrual cycle irregularity and metabolic disorders: a population-based prospective study. PLoS One 2016 Dec 16; 11 (12): e0168402 [Full Text]
- Patra S, Sagar S. Effect of diet on evolutionary obstetrics. Interv Gynecology Women’s Healthcare 2018; 3 (1): doi: 10.32474/IGWHC.2018.03.000151.
DISCLAIMER:
The information provided on FX Medicine is for educational and informational purposes only. The information provided on this site is not, nor is it intended to be, a substitute for professional advice or care. Please seek the advice of a qualified health care professional in the event something you have read here raises questions or concerns regarding your health.





