N-Palmitoylethanolamide (PEA), is an endogenously produced lipid1 found in the plasma membrane2 with concentrations increasing in response to tissue damage, inflammation, and nociceptive fibre stimulation.1 Dietary sources include egg yolks, soy lecithin, bovine and human milk, roasted coffee, apples, potatoes, lentils, black-eyed peas, tomatoes, corn, peanuts, common beans, garden peas, and soybeans.3
Initially positioned as a supplement for the prevention of influenza and the common cold,2 and treatment of rheumatic fever,2,4 today PEA is regarded for its anti-inflammatory, analgesic, immunomodulatory, antimicrobial, and neuroprotective effects with the ability to effect numerous pathways and sites within the body.5

The musculoskeletal system
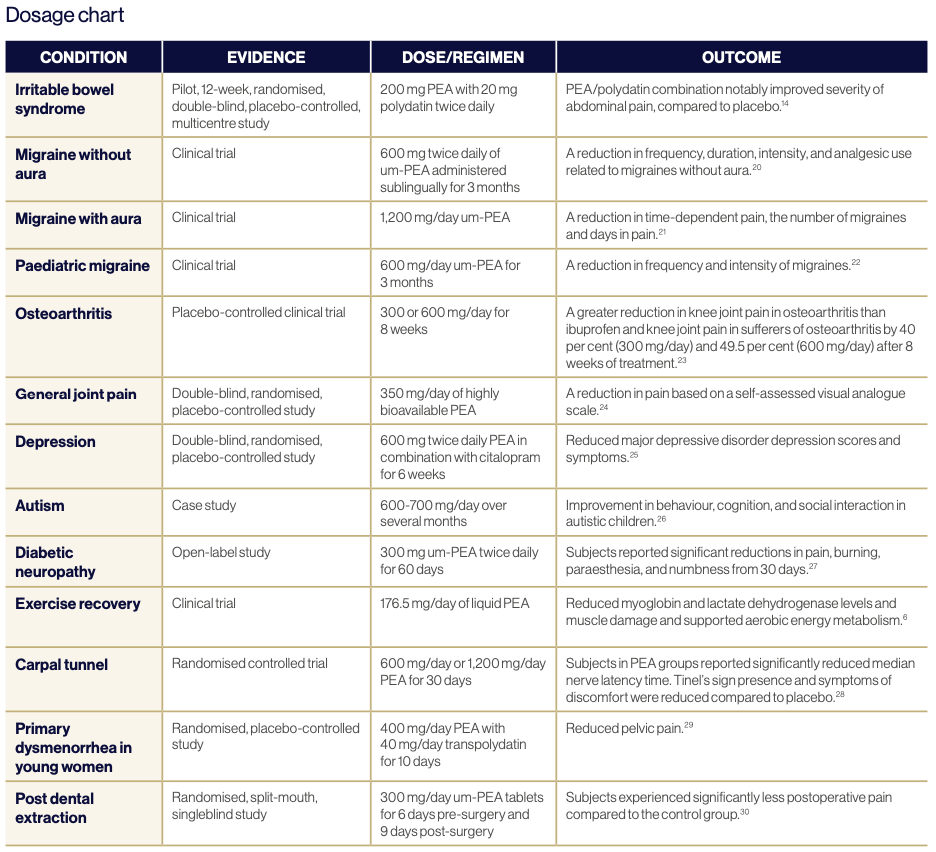
From a lifestyle perspective, PEA supports exercise recovery and may reduce exercise withdrawal due to pain and loss of strength from inflammation. It also supports individuals looking to increase their strength, fitness, and overall health.5 In clinical trials, PEA reduced muscle damage, supported aerobic energy metabolism, and reduced lactate levels to encourage protein synthesis.6
PEA may reduce pain and inflammation in joints, with studies showing arthritis and rheumatoid arthritis joints had lower PEA levels in the synovial fluid.1 Meanwhile, PEA levels increased in chronic neck and shoulder pain subjects to reduce pain and inflammation, however, PEA levels did not increase in chronic widespread pain subjects suggesting a potential for PEA supplementation.7 Carpal tunnel syndrome patients demonstrated an objective improvement in sleep quality and associated pain intensity,1 while animal studies on fracture pain demonstrate PEA improved fracture repair.2
The nervous system
PEA reduces pain, stress, depression, and anxiety, as well as supporting post-traumatic stress disorder. It has shown to support γ-aminobutyric acid function and reduce animal seizure activity via allopregnanolone production.2 Concomitant use with analgesics and PEA has demonstrated pain reduction and decreased medication reliance.1 It is observed endogenous PEA levels rise in acute situations, with chronic demand potentially exhausting PEA levels, warranting exogenous supplementation.5
In animal models, PEA has shown to reduce mast cell- induced neuroinflammation, demyelination and neuropathic pain. Additionally, it functions to lower mast cell-mediated toxicity, brain oedema and ischaemia, delayed post glutamate excitotoxic neuronal death, depression, anhedonia, and amyloid γ-peptide-induced learning and memory impairment2 in neurodegenerative disorders.5 Parkinson’s disease patients prescribed ultra-micronised PEA (um-PEA) with levodopa demonstrated motor and nonmotor symptom improvement2 while multiple sclerosis clinical studies support a reduction in pain with increased plasma PEA levels.2,8
A clinical study using PEA in conjunction with risperidone demonstrated an improvement in irritability and hyperactivity in autistic patients2 while reduced dopamine rewarding effects of nicotine and cocaine were determined in animal studies,9 suggesting a potential therapeutic role for autism and addiction support. Observational studies on stroke patients identified improved cognitive function, spasticity, and ability following PEA administration,10 with circulation levels at the time of the stroke correlating with the degree of neurological impact.11

The immune system
Preclinical studies demonstrate PEA’s bacterial and viral resistance to infections through innate immune support by binding to macrophages and mast cells, supporting inflammatory cytokine modulation.5
Mast cell modulation by PEA inhibits histamine, prostaglandin D2, and tumour necrosis factor-a release, leading to the prevention of allergic rhinitis, dermatitis, asthma, and wheals that involve mast cell degranulation and inflammation.5 Topical application of PEA demonstrates a reduction of eczema.12
The gastrointestinal system
PEA is a ligand for cannabinoid-like-G-coupled receptors GPR55 and GPR119. The GPR55 receptor is expressed throughout the body although mainly in the gastrointestinal tract, frontal cortex, hippocampus, hypothalamus, cerebellum, and brainstem,2 whereas the GPR119 receptor is predominantly expressed in the gut and pancreas. Connections have been made between increased GPR119 expression and type 2 diabetes, obesity, and metabolic disorders.1 Mice with diabetic neuropathy demonstrated increased cutaneous PEA levels following irritation and inflammation.13
Gastrointestinal GPR55 expression modulates inflammation, gastric motility, secretion, cellular proliferation, and intestinal permeability.2 Gastrointestinal dysbiosis correction is enhanced with PEA increasing commensal bacteria including Akkermansia muciniphila, Eubacterium, and Enterobacteriaceae.5 Intestinal lipopolysaccharides promote inflammation and intestinal hyperpermeability, crossing the blood-brain-barrier where they promote neuroinflammation. PEA reduces the impact of lipopolysaccharides by preserving the gastrointestinal barrier integrity5 and stimulates the ligand-activated nuclear receptor Peroxisome proliferator-activated receptor-α (PPAR-α) subtype involved in the regulation of macronutrient metabolism.3
Clinical studies of ulcerative colitis demonstrated intestinal PEA levels 1.8 times greater than healthy controls,13 and two times greater for untreated coeliac disease patients, while pancreatitis, pancreatic cancer, cirrhosis, and colonic inertia also increase localised PEA.2 Studies on irritable bowel syndrome showed reduced symptoms14 while an increase in intestinal PEA reduced local and systemic inflammation in colitis-induced mice indicating therapeutic potential.2
The reproductive system
PEA reduced pelvic pain associated with painful bladder syndrome, dysmenorrhea, and endometriosis following PEA administration.15 PEA prevents mast cell recruitment, degranulation, and inflammation by downregulating COX-2, reducing pain associated with menstruation, hormonal fluctuation, and endometrial lesions.5 PEA combined with transpolydactin reduced dysmenorrhea, dyspareunia, and endometriosis symptoms, providing patients with a reduction in pain in a murine model.2

Toxicity and adverse events
An animal toxicity study found a no-observed-adverse-effect level of PEA for maternal toxicity, embryotoxicity, fetotoxicity, and teratotoxicity greater than 1,000 mg/kg body weight/day suggesting a human equivalent of 9.7 g/day.17
Patients with renal and hepatic impairment can safely use PEA as its metabolism is localised and cellular.18
Clinical and animal trials using PEA have not identified any adverse drug-drug interaction and adverse effects have been limited to occasional stomach upset or diarrhoea2 with PEA being well-tolerated.5
Download a pdf copy of this article
References
1 Cordaro M, Cuzzocrea S, Crupi R. An Update of Palmitoylethanolamide and Luteolin Effects in Preclinical and Clinical Studies of Neuroinflammatory Events. Antioxidants. 2020 Mar 5;9(3):216.
2 Davis MP, Behm B, Mehta Z, Fernandez C. The Potential Benefits of Palmitoylethanolamide in Palliation: A Qualitative Systematic Review. The American Journal of Hospice & Palliative Care [Internet]. 2019 Dec 1 [cited 2022 Mar 7];36(12):1134–54. Available from: https://pubmed.ncbi.nlm.nih.gov/31113223/
3 Noce A, Albanese M, Marrone G, Di Lauro M, Pietroboni Zaitseva A, Palazzetti D, et al. Ultramicronized Palmitoylethanolamide (um-PEA): A New Possible Adjuvant Treatment in COVID-19 patients. Pharmaceuticals. 2021 Apr 6;14(4):336.
4 Coburn A. The Concept of Egg Yolk as a Dietary Inhibitor to Rheumatic Susceptibility. The Lancet. 1960 Apr 1;275(7129):867–70.
5 Clayton P, Hill M, Bogoda N, Subah S, Venkatesh R. Palmitoylethanolamide: A Natural Compound for Health Management. International Journal of Molecular Sciences [Internet]. 2021 May 18 [cited 2022 Apr 2];22(10):5305. Available from: https://www.
ncbi.nlm.nih.gov/pmc/articles/PMC8157570/
6 Mallard A, Briskey D, Richards A, Mills D, Rao A. The Effect of Orally Dosed Levagen+TM (palmitoylethanolamide) on Exercise Recovery in Healthy Males—A Double-Blind, Randomized, Placebo-Controlled Study. Nutrients. 2020 Feb 25;12(3):596.
7 Ghafouri N, Ghafouri B, Larsson B, Stensson N, Fowler CJ, Gerdle B. Palmitoylethanolamide and stearoylethanolamide levels in the interstitium of the trapezius muscle of women with chronic widespread pain and chronic neck-shoulder pain correlate with pain intensity and sensitivity. Pain. 2013 Sep;154(9):1649–58.
8 Orefice NS, Alhouayek M, Carotenuto A, Montella S, Barbato F, Comelli A, et al. Oral Palmitoylethanolamide Treatment Is Associated with Reduced Cutaneous Adverse Effects of Interferon-β1a and Circulating Proinflammatory Cytokines in Relapsing–Remitting Multiple Sclerosis. Neurotherapeutics. 2016 Feb 8;13(2):428–38.
9 Hansen HS. Palmitoylethanolamide and other anandamide congeners. Proposed role in the diseased brain. Experimental Neurology. 2010 Jul;224(1):48–55.
10 Caltagirone C, Cisari C, Schievano C, Rosanna Di Paola, Cordaro M, Bruschetta G, et al. Co-ultramicronized Palmitoylethanolamide/Luteolin in the Treatment of Cerebral Ischemia: from Rodent to Man. 2016 Feb 1;7(1):54–69.
11 Naccarato M, Pizzuti D, Petrosino S, Simonetto M, Ferigo L, Grandi F, et al. Possible Anandamide and Palmitoylethanolamide involvement in human stroke. Lipids in Health and Disease. 2010;9(1):47.
12 Eberlein B, Eicke C, Reinhardt H-W, Ring J. Adjuvant treatment of atopic eczema: assessment of an emollient containing N-palmitoylethanolamine (ATOPA study). Journal of the European Academy of Dermatology and Venereology. 2007 Jul 10;0(0):070806223155012
13 Darmani NA, Izzo AA, Degenhardt BF, Valenti M, Scaglione G, Capasso R, et al. Involvement of the cannabimimetic compound, N-palmitoyl-ethanolamine, in inflammatory and neuropathic conditions: Review of the available pre-clinical data, and first human studies. 2005 Jun 1;48(8):1154–63.
14 Cremon C, Stanghellini V, Barbaro MR, Cogliandro RF, Bellacosa L, Santos J, et al. Randomised clinical trial: the analgesic properties of dietary supplementation with palmitoylethanolamide and polydatin in irritable bowel syndrome. Alimentary Pharmacology & Therapeutics. 2017 Feb 6;45(7):909–22.
15 Stochino-Loi E, Pontis A, Cofelice V, Pirarba S, Fais MF, Daniilidis A, et al. Effect of ultramicronized-palmitoylethanolamide and co-micronized palmitoylethanolamide/polydatin on chronic pelvic pain and quality of life in endometriosis patients: An openlabel pilot study. International Journal of Women’s Health. 2019 Aug;Volume 11:443–9
16 Beggiato S, Tomasini MC, Ferraro L. Palmitoylethanolamide (PEA) as a Potential Therapeutic Agent in Alzheimer’s Disease. Frontiers in Pharmacology. 2019 Jul 24;10.
17 Deshmukh NS, Gumaste S, Subah S, Bogoda NO. Palmitoylethanolamide: Prenatal Developmental Toxicity Study in Rats. International Journal of Toxicology [Internet]. 2021 Feb 12 [cited 2023 Jan 22];40(2):161–70. Available from: https://www.ncbi.
nlm.nih.gov/pmc/articles/PMC7961647/
18 Hesselink JK, Kopsky DJ. The Role of Palmitoylethanolamide, an Autacoid, in the Symptomatic Treatment of Muscle Cramps: Three Case Reports and Review of Literature. Journal of Clinical Case Reports. 2016;06(03).
19 Esposito G, Capoccia E, Turco F, Palumbo I, Lu J, Steardo A, et al. Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-α activation. Gut. 2013 Sep 30;63(8):1300–12.
20 Dalla Volta G. Ultramicronized palmitoylethanolamide reduces frequency and pain intensity in migraine. A pilot study. International Journal of Neurology and Brain Disorders. 2016;3(2):1–5.
21 Chirchiglia D, Cione E, Caroleo MC, Wang M, Di Mizio G, Faedda N, et al. Effects of Add-On Ultramicronized N-Palmitol Ethanol Amide in Patients Suffering of Migraine With Aura: A Pilot Study. Frontiers in Neurology. 2018 Aug 17;9.
22 Papetti L, Sforza G, Tullo G, Alaimo di Loro P, Moavero R, Ursitti F, et al. Tolerability of Palmitoylethanolamide in a Pediatric Population Suffering from Migraine: A Pilot Study. Pain Research and Management. 2020 Apr 24;2020:1–7.
23 Steels E, Venkatesh R, Steels E, Vitetta G, Vitetta L. A double-blind randomized placebo controlled study assessing safety, tolerability and efficacy of palmitoylethanolamide for symptoms of knee osteoarthritis. Inflammopharmacology. 2019 Mar 29;
24 Briskey D, Roche G, Rao A. The Effect of a Dispersible Palmitoylethanolamide (Levagen+) Compared to a Placebo for Reducing Joint Pain in an Adult Population – A Randomised, Double-Blind Study. International Journal of Nutrition and Food Sciences. 2021;10(1):9.
25 Ghazizadeh-Hashemi M, Ghajar A, Shalbafan MR, Ghazizadeh-Hashemi F, Afarideh M, Malekpour F, et al. Palmitoylethanolamide as adjunctive therapy in major depressive disorder: A double-blind, randomized and placebo-controlled trial. Journal of Affective Disorders. 2018 May;232:127–33.
26 Antonucci N, Cirillo A, Siniscalco D. Beneficial Effects of Palmitoylethanolamide on Expressive Language, Cognition, and Behaviors in Autism: A Report of Two Cases. Case Reports in Psychiatry. 2015;2015:1–6.
27 Schifilliti C, Cucinotta L, Fedele V, Ingegnosi C, Luca S, Leotta C. Micronized Palmitoylethanolamide Reduces the Symptoms of Neuropathic Pain in Diabetic Patients. Pain Research and Treatment. 2014 Apr 2;2014:1–5.
28 Conigliaro R, Drago V, Foster PS, Schievano C, Di Marzo V. Use of palmitoylethanolamide in the entrapment neuropathy of the median in the wrist. Minerva Medica [Internet]. 2011 Apr 1 [cited 2024 Feb 27];102(2):141–7. Available from: https://pubmed.ncbi.nlm.nih.gov/21483401/
29 Tartaglia E, Armentano M, Giugliano B, Sena T, Giuliano P, Loffredo C, et al. Effectiveness of the Association N-Palmitoylethanolamine and Transpolydatin in the Treatment of Primary Dysmenorrhea. Journal of Pediatric and Adolescent Gynecology. 2015 Dec;28(6):447–50.
30 Bacci C, Cassetta G, Emanuele B, Berengo M. Randomized Split-Mouth Study on Postoperative Effects of Palmitoylethanolamide for Impacted Lower Third Molar Surgery. ISRN Surgery. 2011;2011:1–6.


